A
B
C
D
Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
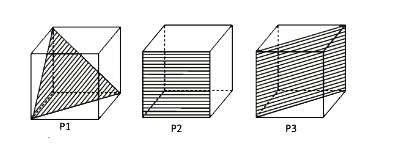
- Following three planes (P1, P2, P3) in an FCC unit cell are shown. Con...
Text Solution
|
- If p1,p2,p3 are respectively the perpendicular from the vertices of a ...
Text Solution
|
- Following three planes (P(1), P(2), P(3)) in an fcc unit cell are show...
Text Solution
|
- Which of the following statements is/are correct about TVs in an fcc u...
Text Solution
|
- P1 and P2 are respectively :
Text Solution
|
- The below figure shows 2 plants of the same species identify the types...
Text Solution
|
- Following three planes (P1, P2, P3) in an FCC unit cell are shown. Con...
Text Solution
|
- Consider the following reaction A to P1, B to P2 , C to P3 , D to P4 ,...
Text Solution
|
- p1^x1 * p2^x2 * p3^x3 * p4^x4 = 113400 இங்கு p1, p2, p3, p4 என்பன ஏறு ...
Text Solution
|