A
B
C
D
Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
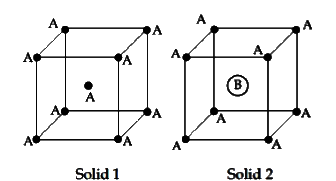
- Consider the bcc unit cells of the solids 1 and 2 with the position of...
Text Solution
|
- Consider the bcc unit cells of the solids 1 and 2 with the position of...
Text Solution
|
- In a body-centred cubic unit cell of closest packed atoms, the radius ...
Text Solution
|
- In the primitie cubic unit cell of closed packed atoms, the radius of ...
Text Solution
|
- In the face-centred cubic unit cell of cloest packed atom,s the radiu...
Text Solution
|
- In a face centred cubic unit cell of close packed atoms, the radius of...
Text Solution
|
- In a face centred cubic unit cell of close packed atoms, the radius of...
Text Solution
|
- ठोस 1 तथा 2 परमाणुओं की स्थिति के साथ, जैसा कि नीचे दर्शाया गया है, की...
Text Solution
|
- Metallic chromium crystallises in bcc lattice. The edge length of unit...
Text Solution
|