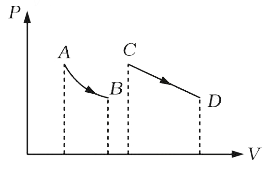
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Two adiabatic expansions of n mole of same gas are shown. If VB/VA = V...
Text Solution
|
- One mole of a diatomic ideal gas (gamma=1.4) is taken through a cyclic...
Text Solution
|
- In uniform electric field E=10 N//C , find a. VA-VB , b. VB-VC
Text Solution
|
- In the uniform electric field shown in figure, find : a. VA-VD b....
Text Solution
|
- Find out the following a. VA - VB b. VB - VC c. VC - VA d. VD...
Text Solution
|
- Two adiabatic expansions of n mole of same gas are shown. If VB/VA = V...
Text Solution
|
- दो आवेश +q तथा-q चित्रानुसार व्यवस्थित हैं। A तथा B बिन्दुओं पर विभव क...
Text Solution
|
- One mole of a diatomic ideal gas ( gamma = 1.4) is taken through a cyc...
Text Solution
|
- In uniform electric field E=10 N//C, find a. VA-VB , b. VB-VC
Text Solution
|