A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
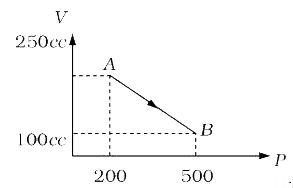
- A mono-atomic gas is taken along path AB as shown. Calculate change in...
Text Solution
|
- A gas is taken along the path AB as shown in , if 70 cal of heat is ex...
Text Solution
|
- In the diagrams (i) to (iv) of variation of volume with changing press...
Text Solution
|
- A mono-atomic gas is taken along path AB as shown. Calculate change in...
Text Solution
|
- One mole of an ideal diatomic gas undergoes a transition from A to B a...
Text Solution
|
- One mole of an ideal diatomic gas undergoes a transition from A to B a...
Text Solution
|
- एक आदर्श गैस को, चित्र में दर्शाये गये अनुसार चक्रीय प्रक्रम abca से ग...
Text Solution
|
- One mole of an ideal diatomic gas undergoes a transition from A to B a...
Text Solution
|
- एक आदर्श गैस को, चित्र में दर्शाये गये अनुसार चक्रीय प्रक्रम abca से ग...
Text Solution
|