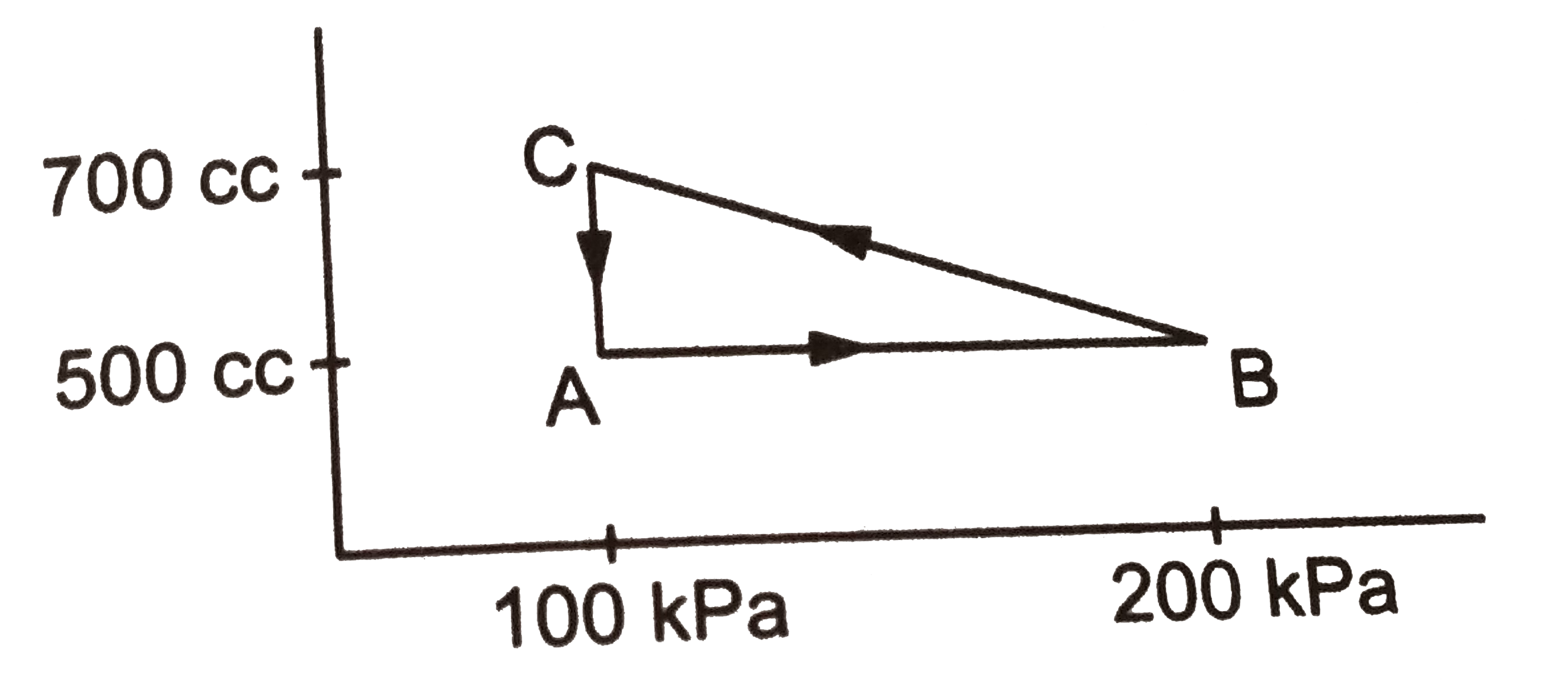
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- A gas is taken through a cyclic process ABCA as shown in, if 2.4 cal o...
Text Solution
|
- A gas is taken through a cyclic process ABCA as shown in, if 2.4 cal o...
Text Solution
|
- A substance is taken through the process abc as shown in, if the inte...
Text Solution
|
- In given figure, An ideal gas a gas is taken through a cyclic process ...
Text Solution
|
- A thermodynamics system is taken through the cyclic process abca as sh...
Text Solution
|
- An ideal monatomic gas undergoes a cyclic process ABCA as shown in the...
Text Solution
|
- A sample of helium is undergoing a cyclic process ABCA as shown. In wh...
Text Solution
|
- One mole of monoatomic gas taken through a cyclic process as shown in ...
Text Solution
|
- Corresponding to the process shown in figure, the heat given to the ga...
Text Solution
|