A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Recommended Questions
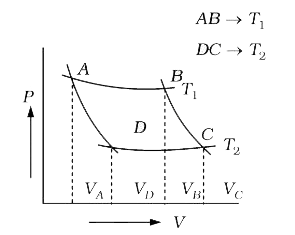
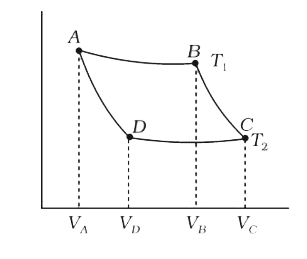
- In the following P–V diagram of an ideal gas, two adiabates cut two is...
Text Solution
|
- In the following P-V diagram two adiabatics cut two isothermals at tem...
Text Solution
|
- In the following P–V diagram of an ideal gas, two adiabates cut two is...
Text Solution
|
- দুটি ভিন্ন তাপমাত্রা T1 এবং T2 -তে ( T2gtT1 ) নির্দিষ্ট ভরের একটি আদর্...
Text Solution
|
- Figure shows graphs of pressure versus density for an ideal gas at two...
Text Solution
|
- दो भिन्न तापों T1 तथा T2 (T1 lt T2) पर एक बन्द निकाय में एक आदर्श गैस ...
Text Solution
|
- Two different temperatures T1 And T2 (T1 lt T2) But consider reversibl...
Text Solution
|
- Diagram shows a graph between pressure and density for an ideal gas at...
Text Solution
|
- Diagram shows a graph between pressure and density for an ideal gas at...
Text Solution
|