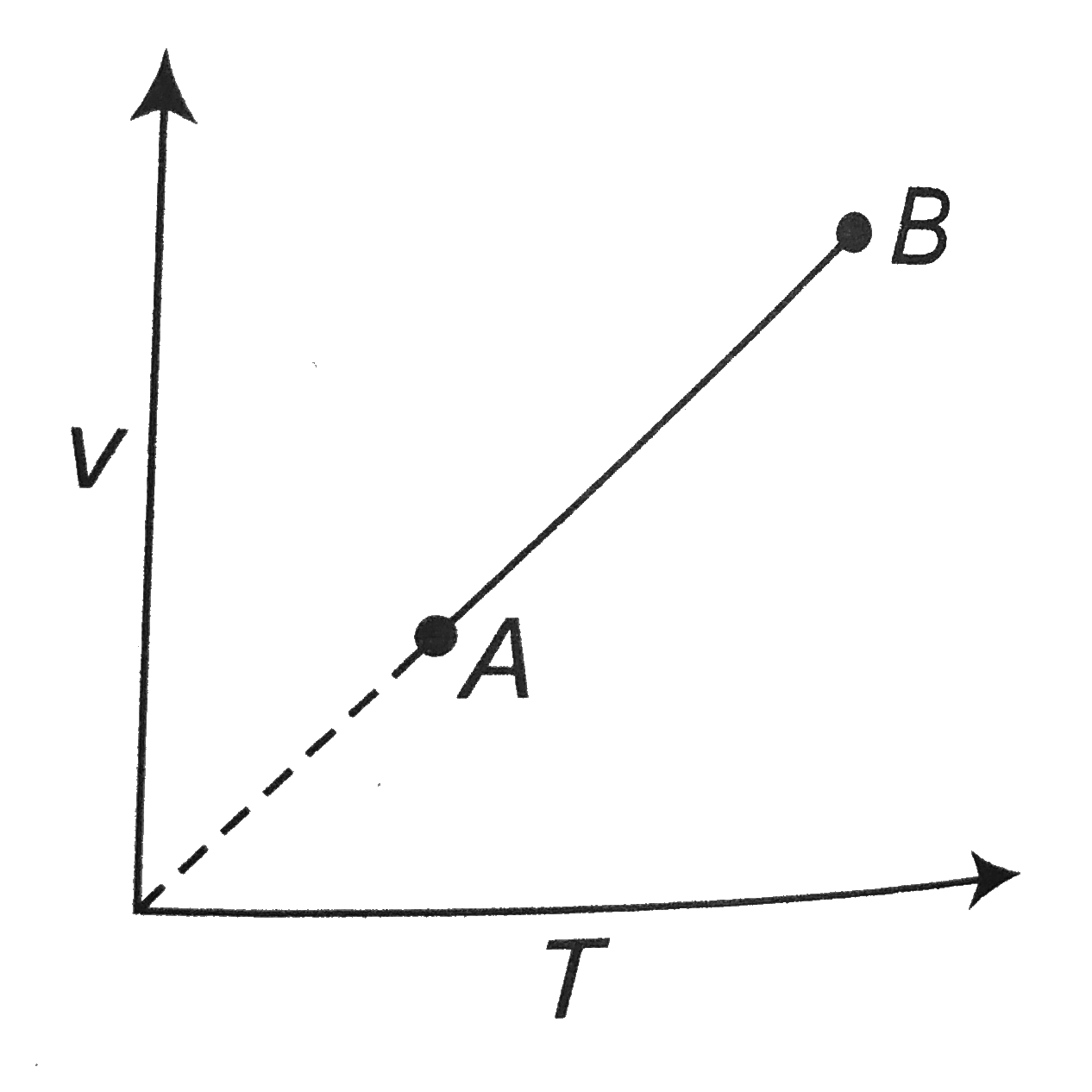
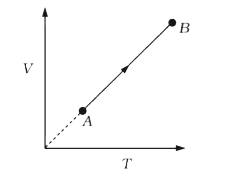
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- An ideal monoatomic gas undergoes the process AB as shown in the figur...
Text Solution
|
- Find the ratio of (DeltaQ)/(DeltaU) and (DeltaQ)/(DeltaW) in an isobar...
Text Solution
|
- Heat is supplied to a diatomic gas at constant pressure. The ratio of ...
Text Solution
|
- An ideal monoatomic gas undergoes the process AB as shown in the figur...
Text Solution
|
- An ideal monoatomic gas undergoes a cyclic process ABCA as shown in th...
Text Solution
|
- Two moles of an ideal monoatomic gas undergoes a process VT = constant...
Text Solution
|
- Two moles of a monoatomic ideal gas undergoes a process AB as shown in...
Text Solution
|
- The figures given below show different processes (relating pressure P ...
Text Solution
|
- Two moles of an ideal monoatomic gas undergoes a cyclic process ABCA a...
Text Solution
|