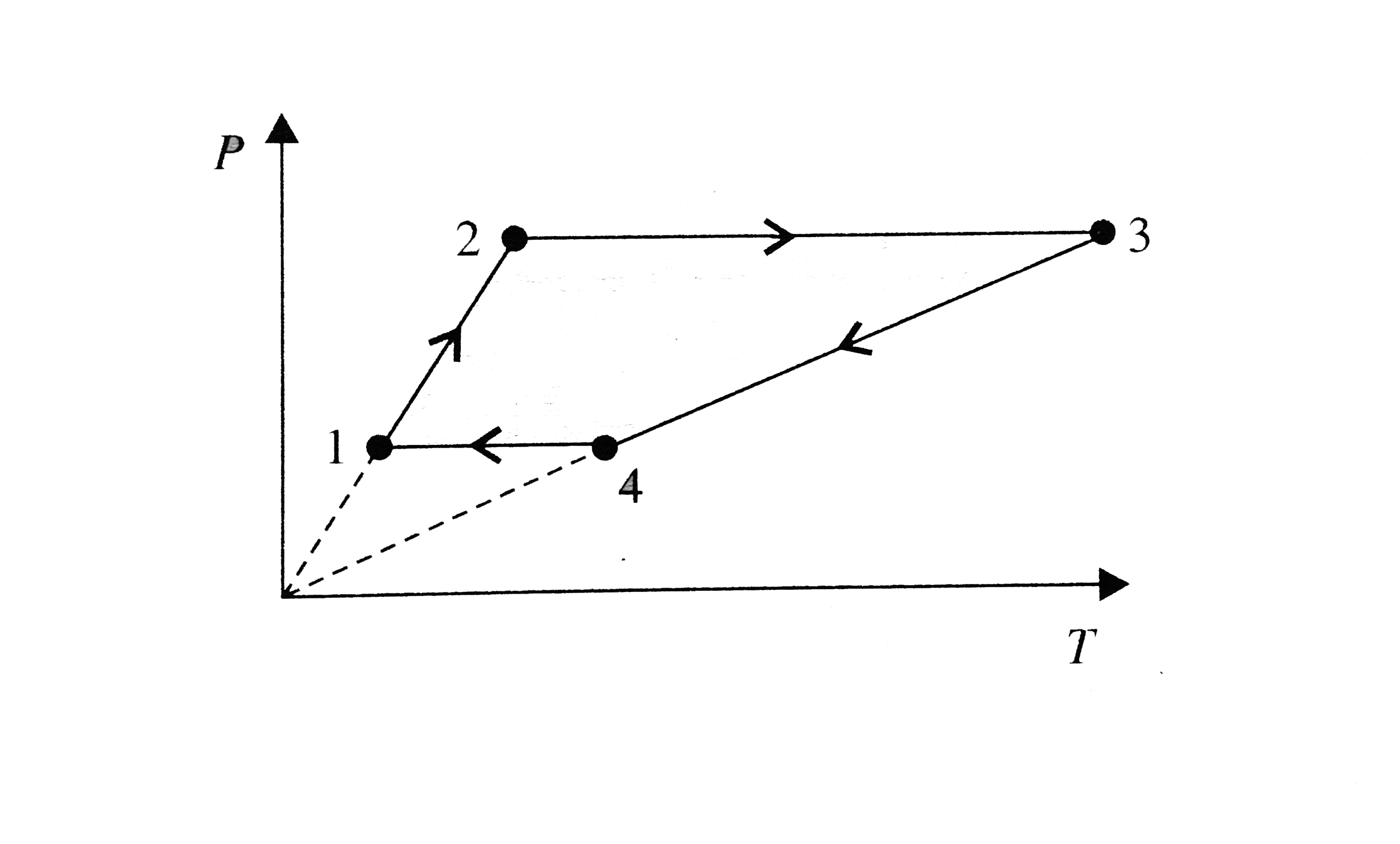
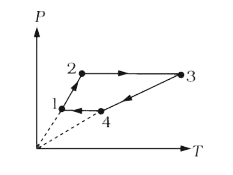
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Three moles of an ideal monoatomic gas perform a cycle shown in figure...
Text Solution
|
- The moles of an ideal monoatomic gas perform a cycle shown in figure. ...
Text Solution
|
- A monatomic gas undergoes a cycle consisting of two isothermals and tw...
Text Solution
|
- A monatomic gas undergoes a cycle consisting of two isothermals and tw...
Text Solution
|
- A monatomic gas undergoes a cycle consisting of two isothermals and tw...
Text Solution
|
- Six moles of an ideal gas performs a cycle shown in figure. If the tem...
Text Solution
|
- Three moles of an ideal monoatomic gas per form a cyclic as shown in t...
Text Solution
|
- Three moles of an ideal monoatomic gas per form a cyclic as shown in t...
Text Solution
|
- 3 moles of an ideal mono atomic gas performs a cycle as shown in fig. ...
Text Solution
|