Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Figure shows an ideal gas. Its pressure, volume and temperature are P0...
Text Solution
|
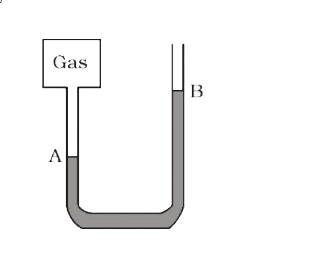
- The heights of mercury surfaces in the tow arms of the manometer shown...
Text Solution
|
- In a constant volume gas thermometer, the pressure of the working gas ...
Text Solution
|
- One of the limbs of a mercury manometer is connected to a gas cylinder...
Text Solution
|
- स्थिर आयतन पर बल्ब में भरी गैस का 0^@C पर दाब 66 सेमी (पारा स्तम्भ) तथ...
Text Solution
|
- A gas at certain volume and temperature has a pressure equal to 75 cm ...
Text Solution
|
- Figure shows an ideal gas. Its pressure, volume and temperature are P0...
Text Solution
|
- A constant volume gas thermometer shows pressure readings of 60 cm and...
Text Solution
|
- The heights of mercury surfaces in the two arms of the manometer shown...
Text Solution
|