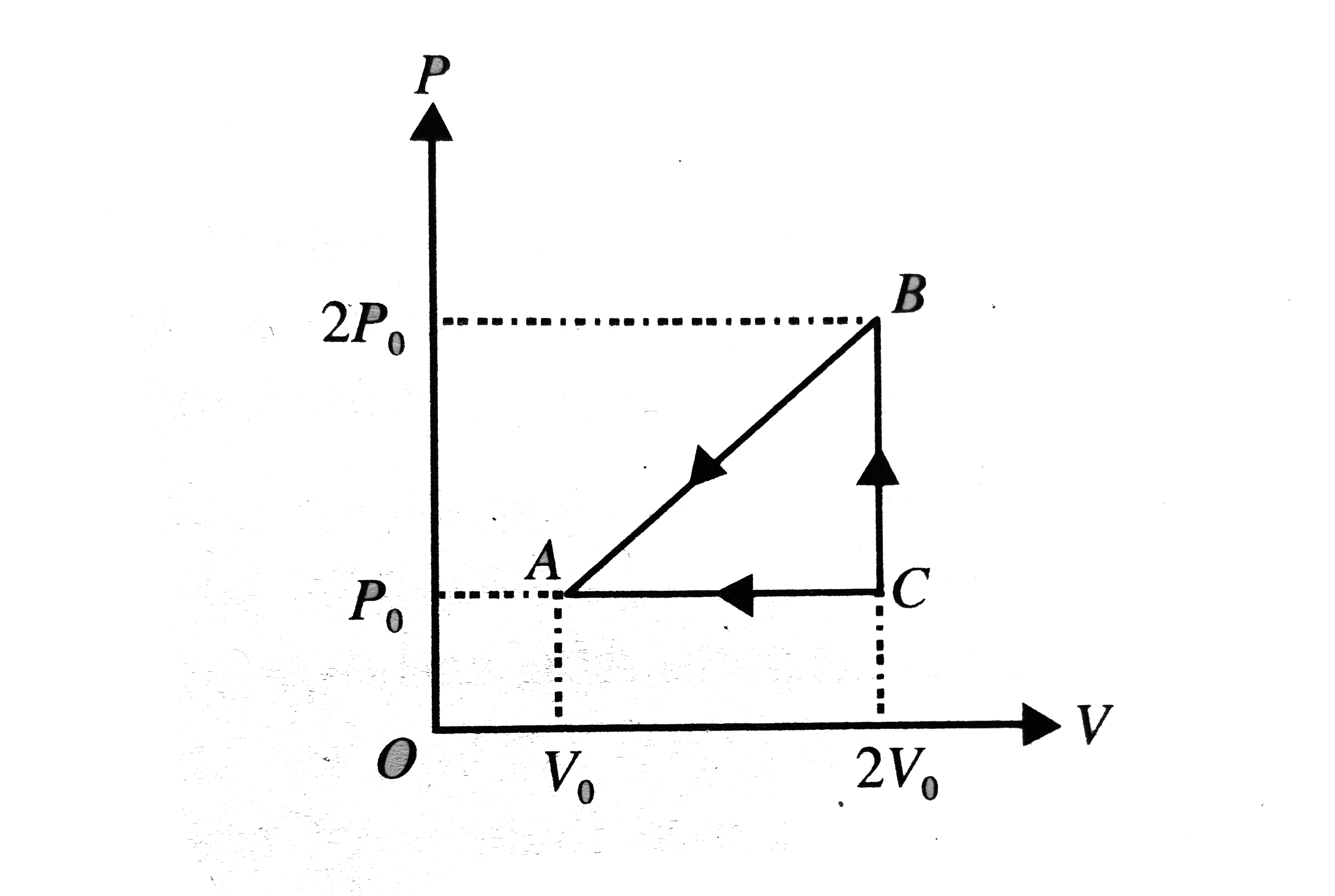
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- One mole of a monatomic gas is taken from a point A to another point B...
Text Solution
|
- One mole of a monatomic gas is taken from a point A to another point B...
Text Solution
|
- A monatomic idea gas of 2 mol is taken through a cyclic process starti...
Text Solution
|
- A monatomic idea gas of 2 mol is taken through a cyclic process start...
Text Solution
|
- A monatomic idea gas of 2 mol is taken through a cyclic process starti...
Text Solution
|
- One mole of an ideal monoatomaic gas is taken from A to C along the pa...
Text Solution
|
- One mole of a gas is in state A[P, V, T(A)] . A small adiabatic proces...
Text Solution
|
- One mole of ideal monatomic gas in taken in cyclice process ABCA a...
Text Solution
|
- One mole of monatomic gas is brought from state A to state B. The init...
Text Solution
|