A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
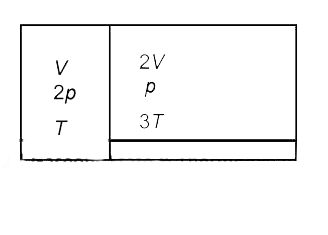
- An ideal gas is kept in two adjacent chambers of volume V and 2V, sepa...
Text Solution
|
- An insulated container of gas has two chambers separated by an insulat...
Text Solution
|
- The chambers of heart are separated by partition called .
Text Solution
|
- An ideal gas is kept in two adjacent chambers of volume V and 2V, sepa...
Text Solution
|
- An insulated container of gas has two chambers separated by an insulat...
Text Solution
|
- One mole of PCl(5) is heated in one litre closed container. If 0.6 mol...
Text Solution
|
- An ideal gas with pressure P, volume V and temperature T is expanded i...
Text Solution
|
- An insulated container of gas has two chambers separated by an insulat...
Text Solution
|
- At 27^@C two moles of an ideal monatomic gas occupies a volume V. The...
Text Solution
|