A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Recommended Questions
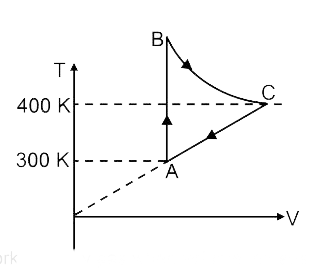
- 3 mole of an ideal gas is taken through the process shown. BC is adiab...
Text Solution
|
- The work done by 1 mole of ideal gas during an adiabatic process is (a...
Text Solution
|
- Three moles of an ideal gas kept at a constant temperature of 300K are...
Text Solution
|
- In figure, a sample of 3 moles of an ideal gas is undergoing through a...
Text Solution
|
- n moles of an ideal gas is taken through a four step cyclic process as...
Text Solution
|
- 50 g of oxygen at N.T.P. is compressed adiabatically to a pressure of ...
Text Solution
|
- 50g of oxygen at NTP is compressed adiabatically to a pressure of 5 at...
Text Solution
|
- 100 mole of an ideal monoatomic gas undergoes a thermodynamic process ...
Text Solution
|
- 3 mole of an ideal gas is taken through the process shown. BC is adiab...
Text Solution
|