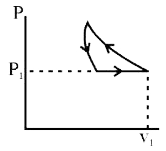
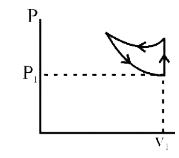
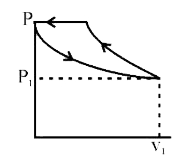
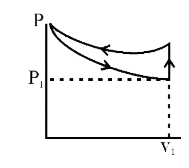
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- A certain mass of an ideal gas is at pressure P(1) and volume V(1). If...
Text Solution
|
- An ideal gas expands isothermally from volume V(1) to V(2) and is then...
Text Solution
|
- A monoatomic gas at pressure P(1) and volume V(1) is compressed adiaba...
Text Solution
|
- A certain mass of an ideal gas is at pressure P(1) and volume V(1) . I...
Text Solution
|
- An ideal gas expands isothermally from volume V(1) to volume V(2) . It...
Text Solution
|
- An ideal gas of volume V and pressure P expands isothermally to volume...
Text Solution
|
- A mass of ideal gas at pressure P is expanded isothermally to four tim...
Text Solution
|
- An ideal gas with pressure P, volume V and temperature T is expanded i...
Text Solution
|
- A monatomic gas at pressure P(1) and volume V(1) is compressed adiabat...
Text Solution
|