A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
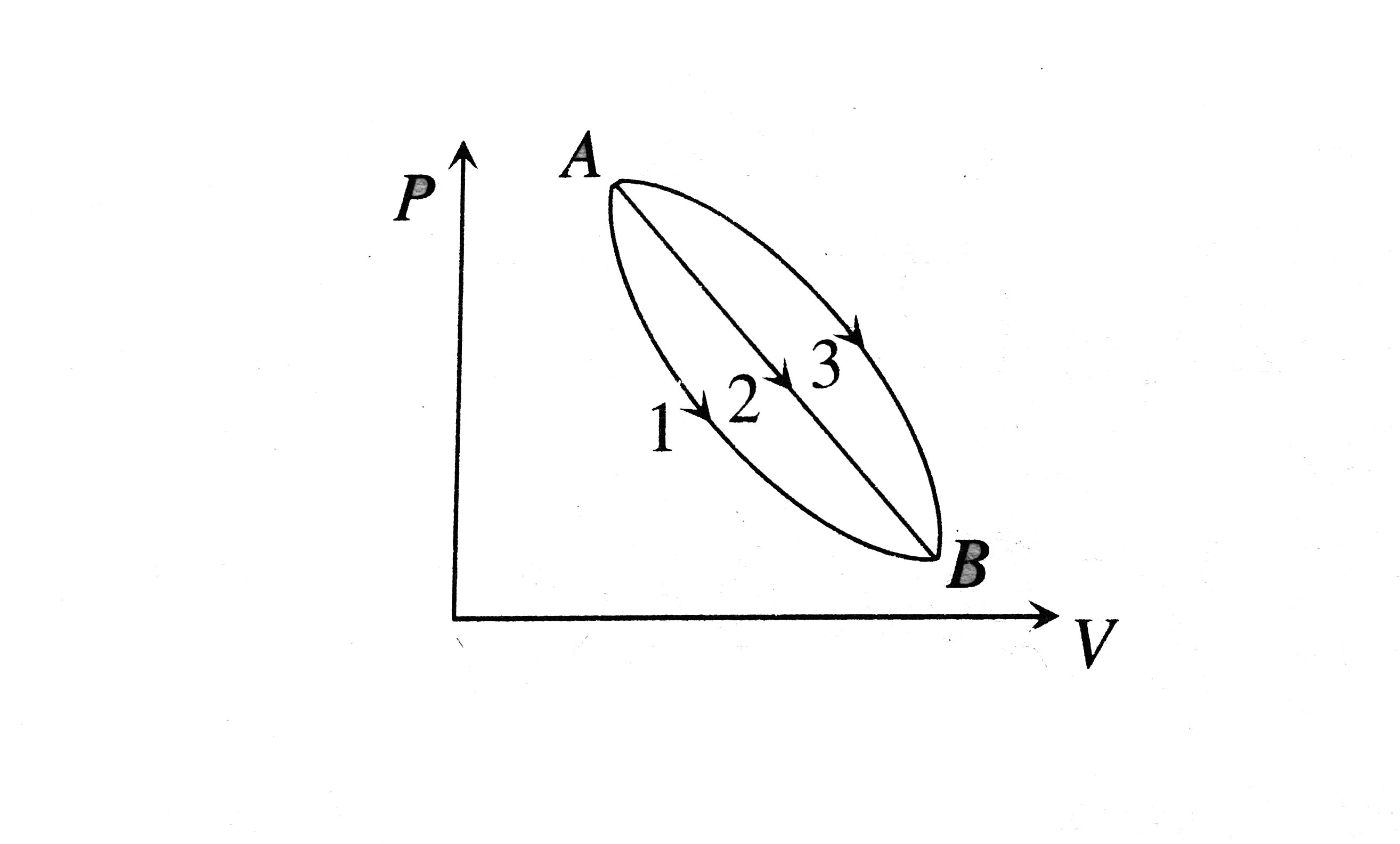
- An ideal gas of mass m in a state A goes to another state B via three ...
Text Solution
|
- Three charged paricles are placed on a straight line as shown in fig. ...
Text Solution
|
- An ideal gas of mass m in a state A goes to another state B via three ...
Text Solution
|
- An ideal gas goes from State A to state B via three different process ...
Text Solution
|
- A system is taken from state A to state B along two different paths 1 ...
Text Solution
|
- For an ideal gas four processes are marked as 1,2,3 and 4 on P-V diagr...
Text Solution
|
- An ideal gas is subjected to cyclic process involving four thermodynam...
Text Solution
|
- दिखाए गए P-V आरेख के अनुसार एक आदर्श गैस को तीन विभिन्न प्रक्रमों द्...
Text Solution
|
- In a process A to B to C to D , the heat absorbed by the system in the...
Text Solution
|