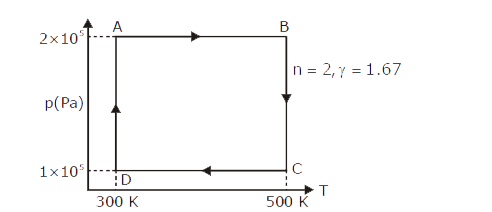
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Assume the gas to be ideal, the work done on the gas in taking it from...
Text Solution
|
- Two moles of helium gas are taken over the cycle ABCDA, as shown in th...
Text Solution
|
- An ideal gas change from state a to state b as shown in Fig. what is t...
Text Solution
|
- Two moles of helium gas are taken over the cycle ABCDA , as sh...
Text Solution
|
- In the adjoining diagram, the p-V graph of an ideal gas is shown. Find...
Text Solution
|
- Assume the gas to be ideal, the work done on the gas in taking it from...
Text Solution
|
- हीलियम गैस के दो मोल चक्र ABCDA पर ले जाते है जैसा कि P-T चित्र में...
Text Solution
|
- An ideal gas is taken in a cyclic process as shown in the figure. Calc...
Text Solution
|
- Three moles of hydrogen gas are compressed isothermally and reversibly...
Text Solution
|