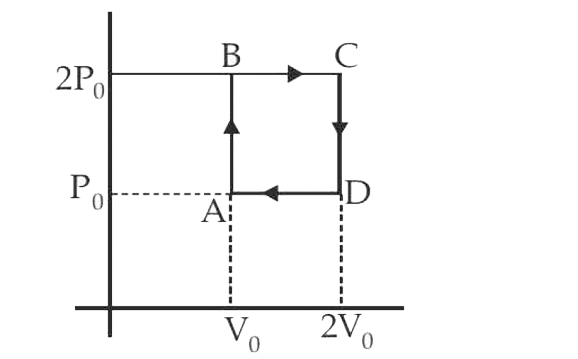
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Helium gas goes through a cycle ABCDA (consisting of two isochoric and...
Text Solution
|
- Helium gas goes through a cycle ABCDA (consisting of two isochoric and...
Text Solution
|
- An ideal monoatomic gas is carried around the cycle ABCDA as shown in ...
Text Solution
|
- An ideal gas whose adiabatic exponent equals gamma goes through a cycl...
Text Solution
|
- One mole of an ideal gas is carried through a thermodynamics cycle as ...
Text Solution
|
- An ideal monoatomic gas is carried around the cycle ABCDA as shown in ...
Text Solution
|
- Two moles of helium gas are taken over the cycle ABCDA , as sh...
Text Solution
|
- हीलियम गैस के दो मोल चक्र ABCDA पर ले जाते है जैसा कि P-T चित्र में...
Text Solution
|
- Helium gas goes through a cycle ABCDA (consisting of two isochoric and...
Text Solution
|