A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
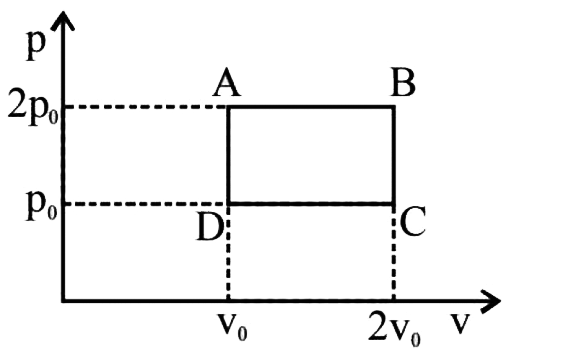
- The above p-v diagram represents the thermodynamic cycle of an engine,...
Text Solution
|
- The above p-v diagram represents the thermodymic cycle of an engine, o...
Text Solution
|
- An ideal monoatomic gas is taken the cycle ABCDA as shown in following...
Text Solution
|
- The figure shows P - V diagram of a thermodynamic cycle Identify the d...
Text Solution
|
- One mole of an ideal monoatomic gas is taken through the thermodynamic...
Text Solution
|
- A Carnot engine used first ideal monoatomic gas and then an ideal diat...
Text Solution
|
- दिया गया p - V चित्र एक आदर्श परमाणुक गैस के साथ कार्य कर रहे एक इंजन ...
Text Solution
|
- An engine operates by taking a monoatomic ideal gas through the cycle ...
Text Solution
|
- আদর্শ একপরমাণুক গ্যাস দ্বারা পরিচালিত একটি ইঞ্জিনের তাপগতীয় চক্র নীচে...
Text Solution
|