A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
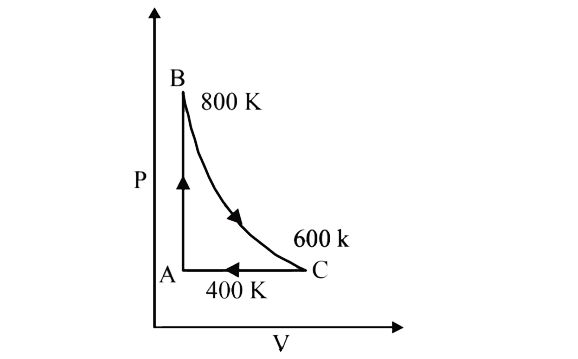
- One mole of a diatomic ideal gas undergoes a cyclic process ABC as sho...
Text Solution
|
- One mole of a diatomic ideal gas undergoes a cyclic process ABC as sho...
Text Solution
|
- A cyclic process of an ideal monoatomic gas is shown in figure. The co...
Text Solution
|
- One mole of an ideal monoatomic gas undergoes a cyclic process as show...
Text Solution
|
- One mole of an ideal monoatomic gas undergoes a cyclic process as show...
Text Solution
|
- One mole of a monatomic ideal gas undergoes a cyclic process as shown ...
Text Solution
|
- One mole of a monatomic ideal gas undergoes a cyclic process as shown ...
Text Solution
|
- One mole of ideal gas undergoes a cyclic process ACBA as shown in figu...
Text Solution
|
- द्विपरमाणुक आदर्श गैस का एक मोल चक्रीय प्रक्रिया ABC से गुजरता है जैसा...
Text Solution
|