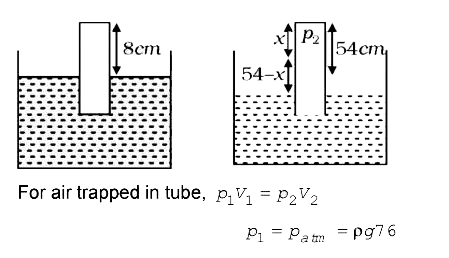
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- An open glass tube is immersed in mercury in such a way that a length ...
Text Solution
|
- An open glass tube is immersed in mercury in such a way that a lenth o...
Text Solution
|
- An open glass tube is immersed in mercury in such a way that a length ...
Text Solution
|
- An open glass tube is immersed in mercury in such a way that a length ...
Text Solution
|
- एक खुली काँच की नाली को पारे में इस प्रकार डुबोया जाता है की पारे की स...
Text Solution
|
- एक खुली काँच नली को पारे में इस प्रकार डुबोया जाता है , की पारे के स्त...
Text Solution
|
- An open glass tube is immersed in mercury in such a way that a length ...
Text Solution
|
- A glass tube open at both ends is immersed vertically in mercury in s...
Text Solution
|
- An open pipe made up of glass is immersed in mercury in such a way tha...
Text Solution
|