A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
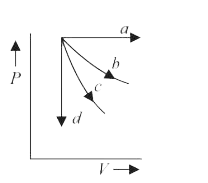
- The given diagram shows four processes i.e., isochoric, isobaric, isot...
Text Solution
|
- PV = n RT holds good for A) Isobaric process B) Isochoric process C) I...
Text Solution
|
- The given diagram shows four processes i.e., isochoric, isobaric, isot...
Text Solution
|
- एक परमाण्विक आदर्श गैस (monatomic ideal gas) का एक मोल (one mole), चार...
Text Solution
|
- दिए गए चित्र में चार प्रक्रम, समआयतनिक, समदाबीय, समतापीय तथा रुद्धोष्म...
Text Solution
|
- What are isobaric, isochoric, isothermal and adiabatic processes?
Text Solution
|
- An ideal gas undergoes four processes: isochoric, isobaric, isothermal...
Text Solution
|
- An ideal gas undergoes four processes: isochoric, isobaric, isothermal...
Text Solution
|
- An ideal gas undergoes four processes: isochoric, isobaric, isothermal...
Text Solution
|