A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
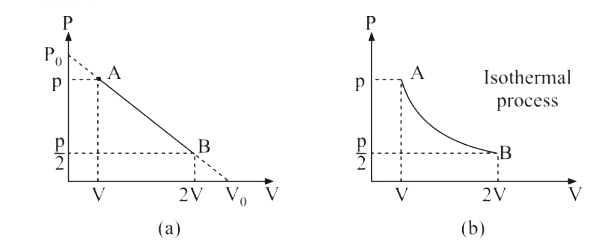
- An ideal gas is taken from the state A (pressure P, volume V) to the s...
Text Solution
|
- An ideal gas is taken from the state A (pressure P, volume V) to the s...
Text Solution
|
- An ideal gas is taken from the state A (pressure p, volume V) to the s...
Text Solution
|
- One mole of an ideal gas goes from an initial state A to final state B...
Text Solution
|
- An ideal gas is taken from the state A (P, V) to the state B (P//2, 2 ...
Text Solution
|
- An ideal gas is taken form the state A(P,V) to the state B((P)/(2),2V)...
Text Solution
|
- An ideal gas taken from state A(P,V) to the state B(P//2,2V) along a s...
Text Solution
|
- STATEMENT-1 : Plot of P vs 1/V (volume) is a straight line for an idea...
Text Solution
|
- An ideal gas is taken from the state A (pressure P, volume V) to the s...
Text Solution
|