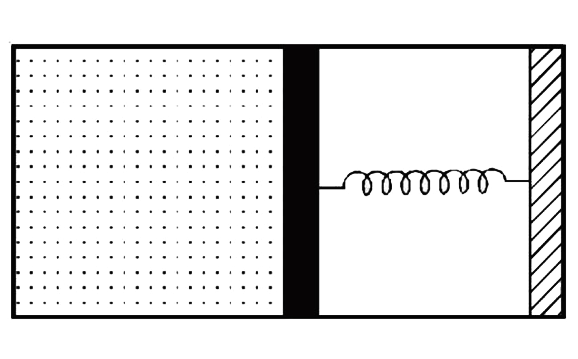
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- An ideal monoatomic gas is confined in a horizontal cylinder by a spri...
Text Solution
|
- An ideal monatonic gas is confined in a cylinder by a spring-loaded pi...
Text Solution
|
- An ideal monoatomic gas is confined in a horizontal cylinder by a spri...
Text Solution
|
- An ideal monatomic gas is confined in a cylinder by a spring-loaded pi...
Text Solution
|
- Two moles of an ideal monoatomic gas are confined within a cylinder by...
Text Solution
|
- A certain mass of an ideal gas at pressure P1 is adiabatically expande...
Text Solution
|
- A gas is filled in a cylinder fitted with a piston at a definite tempe...
Text Solution
|
- एकपरमाणुक आदर्श गैस एक क्षैतिज बर्तन (Horizontal cylinder) में स्प्रिं...
Text Solution
|
- A monoatomic ideal gas, initially at temperature T1 , is enclosed in a...
Text Solution
|