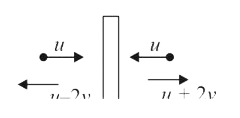
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- A flat plate is moving normal to its plane through a gas under the act...
Text Solution
|
- The average speed of gas molecules is equal to
Text Solution
|
- Assertion : Pressure exerted by gas in a container with increasing tem...
Text Solution
|
- गैस का दाब उसके अणुओं की औसत वर्ग चाल के .......... होता है |
Text Solution
|
- A flat plate is moving normal to its plane through a gas under the act...
Text Solution
|
- The average speed of gas molecules is v at pressure P, If by keeping t...
Text Solution
|
- एक सपाट प्लेट अल्प दबाव के गैस में, अपने तल की अभिलबम्ब दिशा में, बाह्...
Text Solution
|
- एक सपाट प्लेट (flat plate) अलप दबाव के गैस (gas at low pressure) में,...
Text Solution
|
- By how times will the pressure of a gas kept in a gas container of con...
Text Solution
|