A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
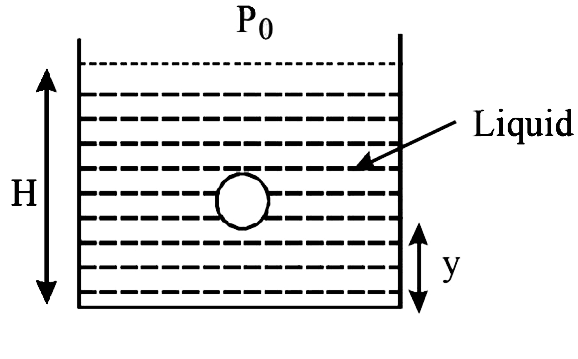
- A small spherical monoatomic ideal gas bubble (gamma=5//3) is trapped ...
Text Solution
|
- A small spherical monoatomic ideal gas bubble (gamma=5//3) is trapped ...
Text Solution
|
- A small spherical monoatomic ideal gas bubble (gamma=5//3) is trapped ...
Text Solution
|
- A small spherical monoatomic ideal gas bubble (gamma=5//3) is trapped ...
Text Solution
|
- A small spherical monoatomic ideal gas bubble (gamma= (5)/(3)) is trap...
Text Solution
|
- A small air bubble of radius r in water is at depth h below the water ...
Text Solution
|
- एकपरमाणुक आदर्श गैस का छोटा बुलबुला (gamma=(5)/(3)), rho(l) घनत्व वाले...
Text Solution
|
- एकपरमाणुक आदर्श गैस का छोटा बुलबुला (gamma=(5)/(3))rho(l) घनत्व वाले द...
Text Solution
|
- एकपरमाणुक आदर्श गैस का छोटा बुलबुला (gamma=(5)/(3))rho(l) घनत्व वाले द...
Text Solution
|