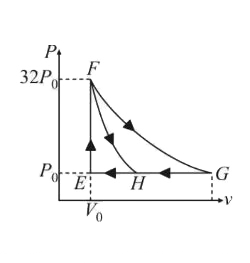
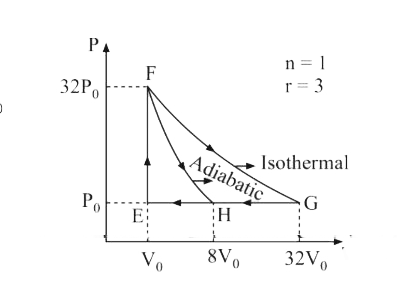
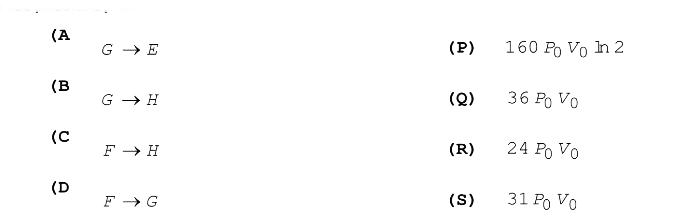Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- One mole of mono-atomic ideal gas is taken along two cyclic processes ...
Text Solution
|
- One mole of an ideal mono-atomic gas is taken round cyclic process ABC...
Text Solution
|
- One mole of a monatomic ideal gas is taken through a cycle ABCDA as s...
Text Solution
|
- Match Column I(reserve area) with Column II (state) and select the cor...
Text Solution
|
- One mole of a monatomic ideal gas is taken along two cyclic processes ...
Text Solution
|
- One mole of mono-atomic ideal gas is taken along two cyclic processes ...
Text Solution
|
- एक एकपरमाणुक आदर्श गैस के एक मोल को, चित्र में दर्शाए pV - आरेख के अनु...
Text Solution
|
- एक एकपरमाणुक आदर्श गैस के एक मोल को चित्र में दर्शाये PV आरेख के अ...
Text Solution
|
- एक एकपरमाणुक आदर्श गैस के एक मोल को चित्र में दर्शाये PV आरेख के अ...
Text Solution
|