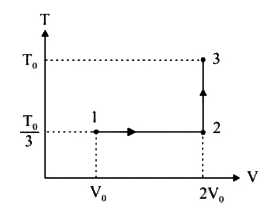
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- In a thermodynamic process on an ideal monatomic gas, the infinitesima...
Text Solution
|
- An ideal monatomic gas expands in such a way that TV^((1)/(2))= consta...
Text Solution
|
- A certain amount of an ideal monoatomic gas undergoes a thermodynamic ...
Text Solution
|
- The ratio of specific heats (C(p) and C(v)) for an ideal gas is gamma....
Text Solution
|
- One mole of a monatomic ideal gas goes through a thermodynamic cycle, ...
Text Solution
|
- In a thermodynamic process on an ideal monatomic gas, the infinitesima...
Text Solution
|
- In a thermodynamic process on an ideal monatomic gas, the infinitesima...
Text Solution
|
- In a thermodynamic process two moles of a monatomic ideal gas obeys PV...
Text Solution
|
- एक आदर्श एकपरमाणुक गैस के एक ऊष्मागतिकी प्रक्रम में गैस द्वारा अतिसूक्...
Text Solution
|