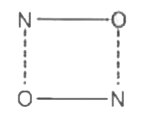
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
TS EAMCET PREVIOUS YEAR PAPERS-TS EAMCET 2019 (6 MAY SHIFT 1)-CHEMISTRY
- The correct order of rates of C-Br bond ionisation of the following br...
Text Solution
|
- If an element having atomic number 96 crystallises in cubic lattice wi...
Text Solution
|
- The vapour pressure of pure C""Cl(4) (molar mass =154gmol^(-1)) and Sn...
Text Solution
|
- A camphor sample melts at 176^(@)C.K(f) for camphor is 40 K kg mol^(-1...
Text Solution
|
- If 'A' is the reactant and 'P' is the product, which one of the follow...
Text Solution
|
- The first order decomposition of H(2)O(2) in an appropriate medium is ...
Text Solution
|
- Which of the following statements is/are not correct? Adsorption is ...
Text Solution
|
- Which one of the following is used to produce Al by electrolysis?
Text Solution
|
- Statement (A) Among the oxides of nitrogen, NO and NO(2) are paramagne...
Text Solution
|
- The products formed during the reaction of carbon with conc. H(2)SO(4)...
Text Solution
|
- How many protons will be consumed when dichromate ion oxidises Fe^(2+)...
Text Solution
|
- Which one of the following is tris (ethane-1, 2-diammine) cobalt (III)...
Text Solution
|
- Which of the following polymer is formed due to the co-polymerisation ...
Text Solution
|
- Find the reagent that oxidises glucose into saccharic acid?
Text Solution
|
- Which one among the following is an antioxidant?
Text Solution
|
- Find the reactants which on heating with alcoholic KOH produces the co...
Text Solution
|
- The major product (P) formed in the below reaction is
Text Solution
|
- Identify X, Y and Z respectively from the following reactions :
Text Solution
|
- Identify the structure of Z in the following reaction sequence Phtha...
Text Solution
|
- The major products P and Q formed in the following reactions of benzon...
Text Solution
|