A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
TS EAMCET PREVIOUS YEAR PAPERS-TS EAMCET 2016-Chemistry
- The order of covalent character of KF, KI
Text Solution
|
- If the kinetic energy in j, of CH(4) (molar mass =16 g mol^(-1)) at T ...
Text Solution
|
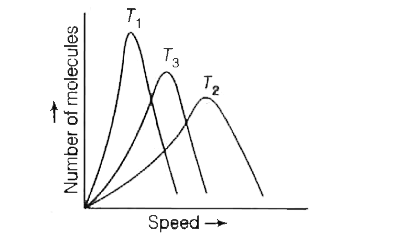
- The given figure shows the maxwell distribution of molecular speeds of...
Text Solution
|
- In Haber's process 50.0g of N(2) [g] and 10.0 g of H(2) [g] are mixed ...
Text Solution
|
- The following reaction occurs in acidic medium KMnP(4) + 8 H^(+) 5e^...
Text Solution
|
- Given N(2)(g)+3H(2)(g)to2NH(3)(g),Delta(r)H^(theta)=-92.4 kJ mol^(-1) ...
Text Solution
|
- Which one of the following is correct?
Text Solution
|
- pH of an aqueous solution of NH(4)CI is
Text Solution
|
- What is the change in the oxidation state of Mn in the reaction of MnO...
Text Solution
|
- Which one of the following will not give flame test?
Text Solution
|
- Which one of the following forms a basic oxide?
Text Solution
|
- The gas produced by the passage of air over hot coke is
Text Solution
|
- In environmental chemistry,the medium which is affected by a pollutant...
Text Solution
|
- The hybridisation of each carbon in the following compound respectivel...
Text Solution
|
- The product Z of the following reaction is H(3)CC -= CH overset(2HBr...
Text Solution
|
- Identify X and Y in the following reaction sequence X overset(Zn)(to...
Text Solution
|
- The packing efficiency of simple cubic (sc.) body centred cubic (bcc) ...
Text Solution
|
- The experimental depression in freezing point of a dilute solution is ...
Text Solution
|
- The molality of an aqueous dilute solution containing non-volatile sol...
Text Solution
|
- Which one of the following is correct polt of vv(m) (in S cm^(2) mol^(...
Text Solution
|