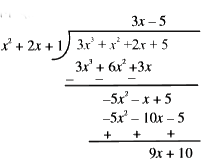Text Solution
Verified by Experts
Topper's Solved these Questions
ORGANIC CHEMISTRY : BASIC PRINCIPLES AND TECHNIQUES
CHHAYA PUBLICATION|Exercise SOLVED WBCHSE SCANNER|38 VideosORGANIC CHEMISTRY : BASIC PRINCIPLES AND TECHNIQUES
CHHAYA PUBLICATION|Exercise SLOVED NCERT EXERCISE|38 VideosORGANIC CHEMISTRY : BASIC PRINCIPLES AND TECHNIQUES
CHHAYA PUBLICATION|Exercise WARM UP EXERICSE|132 VideosMODEL QUESTIONS PAPER
CHHAYA PUBLICATION|Exercise SET III (SECTION II) (GROUP D )|12 VideosP-BLOCK ELEMENTS
CHHAYA PUBLICATION|Exercise PRACTICE SET 7 (ANSWER THE FOLLOWING QUESTIONS) : -|17 Videos
Similar Questions
Explore conceptually related problems
CHHAYA PUBLICATION-ORGANIC CHEMISTRY : BASIC PRINCIPLES AND TECHNIQUES -SHORT ANSWER TYPE
- Arrange cis - but - 2 ene, trans - but - 2 - ene and but - 1 ene in in...
Text Solution
|
- CH(3)Cl is unreactive towards S(N)1 reaction - why?
Text Solution
|
- Explain the orders of acidity of carboxylic acids: (1) Cl(3)C COOHg...
Text Solution
|
- Arrange in order of increasing stability : (1) (CH3)2overset(o+)C H...
Text Solution
|
- Explain why an orgainc liquid vaporises below its boiling point when i...
Text Solution
|
- (1) Write the state of hybridisation of C - atoms menthoned in each of...
Text Solution
|
- The boiling point of a pure organic liquid is 78^(@)C. There are two ...
Text Solution
|
- What is an azeotropic mixture? Give example.
Text Solution
|
- A mixture contains two organic solids, A and B. The solubilities o f A...
Text Solution
|
- What is seeding?
Text Solution
|
- Suggest methods for the separation of the compenents in each of the fo...
Text Solution
|
- A mixture contains three amino acids. How can they be identified?
Text Solution
|
- The R(f) values of X and Y in a mixture determined by TLC method in a ...
Text Solution
|
- Why is an organic compound fused with sodium for testing nitrogen, hal...
Text Solution
|
- (1) The electronic configuration of C - atoms is: 1s^(2)2s^(2)2p^(2), ...
Text Solution
|
- (1) Arrange sp, sp^(2) & sp^(3) - orbitals in increasing order of : ...
Text Solution
|
- Give the IUPAC names of the following compounds: (1) CH(3)CHCICHBrCH...
Text Solution
|
- Write structures of the following: (1) Hept - 5 ene - 1 - yne (2) ...
Text Solution
|
- Draw resonance strutures of following compounds. (1) C(6)H(5)OH (2...
Text Solution
|
- Explain: (1) A mixture of ether and water can be separated by simple d...
Text Solution
|