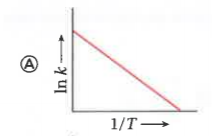
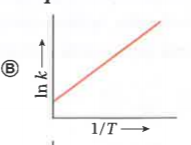
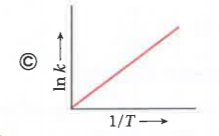
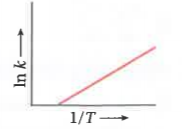
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL KINETICS
CHHAYA PUBLICATION|Exercise SOLVED NCERT EXEMPLAR PROBLEMS (Multiple Choice Question) (More Than One Correct Type)|12 VideosCHEMICAL KINETICS
CHHAYA PUBLICATION|Exercise SOLVED NCERT EXEMPLAR PROBLEMS (Short Answer Type)|20 VideosCHEMICAL KINETICS
CHHAYA PUBLICATION|Exercise ENTRANCE Question Bank (AIIMS)|14 VideosBIOMOLECULES
CHHAYA PUBLICATION|Exercise Practic Set 29|1 VideosCHEMICAL THERMODYNAMICS
CHHAYA PUBLICATION|Exercise Practice Set 6|15 Videos
Similar Questions
Explore conceptually related problems
CHHAYA PUBLICATION-CHEMICAL KINETICS-SOLVED NCERT EXEMPLAR PROBLEMS (Multiple Choice Question) (Single Correct Type)
- The role of a catalyst is to change -
Text Solution
|
- In the presence of a catalyst , the heat evolved or absorbed during th...
Text Solution
|
- Activation energy of a chemical reaction can be determined by-
Text Solution
|
- Consider a first order gas phase decomposition reaction given below : ...
Text Solution
|
- According to Arrhenius equations rate constant (k) is equal to Ae^(-E(...
Text Solution
|
- Consider the Arrhenius equation given below and mark the correct optio...
Text Solution
|
- A graph of volume of hydrogen released vs time for the reaction betwee...
Text Solution
|
- Which of the following statements is not correct about order of a reac...
Text Solution
|
- Consider the graph given in question no. 8 . Which of the following op...
Text Solution
|
- Which of the following statements is not correct -
Text Solution
|
- Which of the following expressions is correct for the rate of reaction...
Text Solution
|
- Rate law for the reaction A+2BrarrC , is found to be Rate =k [A][B]...
Text Solution
|
- Which of following statements is incorrect about the collision theory ...
Text Solution
|
- A first order reaction is 50% completed in 1.26xx10^(14)s. How much ti...
Text Solution
|
- Which of the given statement is incorrect for catalyst-
Text Solution
|
- Value of rate constant of a pseudo first order reaction-
Text Solution
|
- Consider the reaction ArarrB . The concentration of both the reactant...
Text Solution
|