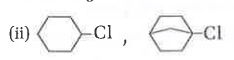Text Solution
Verified by Experts
Topper's Solved these Questions
HALOAKANES AND HALOARENES
CHHAYA PUBLICATION|Exercise Practise set 10 (Choose the correct alternative)|5 VideosHALOAKANES AND HALOARENES
CHHAYA PUBLICATION|Exercise Practise set 10 (Answer the following questions)|10 VideosHALOAKANES AND HALOARENES
CHHAYA PUBLICATION|Exercise Exercise - Fill in the blanks|19 VideosGENERAL PRINCIPLES AND PROCESSES OF ISOLATION OF ELEMENTS
CHHAYA PUBLICATION|Exercise PRACTICE SET 6|10 VideosHYDROCARBONS
CHHAYA PUBLICATION|Exercise PRACTICE SET 13|16 Videos
Similar Questions
Explore conceptually related problems
CHHAYA PUBLICATION-HALOAKANES AND HALOARENES-Exercise - Short answer type questions
- Arrange in the order of increasing S(N)1 reactivity and explain the or...
Text Solution
|
- Solvolysis of (A) in ethanol takes place readily while the solvolysis ...
Text Solution
|
- Which one of each pair will not undergo S(N)2 reaction and why? (i) ...
Text Solution
|
- Which one of each pair will not undergo S(N)2 reaction and why? (ii)...
Text Solution
|
- After standing in aqueous acid, (R)-2-butanol is found to have lost it...
Text Solution
|
- Which one of each pair of compounds will undergo S(N)1 reaction readil...
Text Solution
|
- Which one of each pair of compounds will undergo S(N)1 reaction readil...
Text Solution
|
- In which reaction does recemisation occurs and why?
Text Solution
|
- Prove that in S(N)2 reaction inversion of configuration takes place.
Text Solution
|
- Arrange in increasing priority: -CH(2)CH(3), -CD(2)CH(3), -CH(3),-NH(2...
Text Solution
|
- How can an asymmetric carbon be designated as R or S?
Text Solution
|
- What is the necessary and sufficient condition for a molecule to show ...
Text Solution
|
- What do you mean by enantiomers and diastereoisomers?
Text Solution
|
- What do you mean by meso-compound and racemic modication? Explain why ...
Text Solution
|
- What is Saytzef rule? Explain with an example.
Text Solution
|
- Although haloalkanes can be prepared from alcohols, haloarenes cannot ...
Text Solution
|
- The dipole moment of chlorobenzene is lower than that of cyclohexyl ch...
Text Solution
|
- Why is chlorobenzene inert towards S(N)1 and S(N)2 reactions?
Text Solution
|
- Explain why the melting point of p-dichlorobenzene is higher than that...
Text Solution
|
- Arrange chlorobenzene, p-chloronitrobenzene and m-chloronitrobenzene i...
Text Solution
|