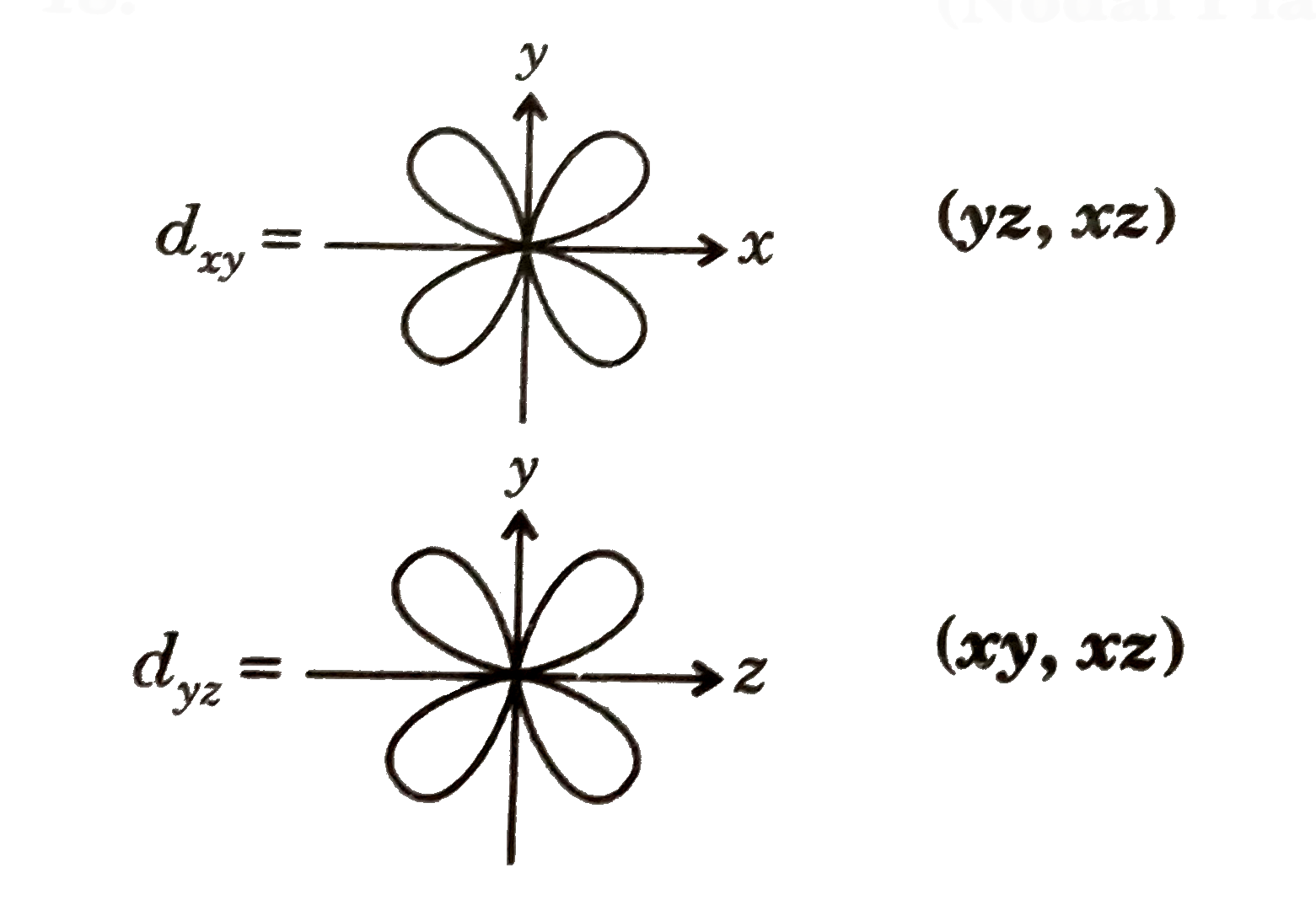A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
QUANTUM NUMBERS AND GENERAL CHEMISTRY
GRB PUBLICATION|Exercise C,|6 VideosQUANTUM NUMBERS AND GENERAL CHEMISTRY
GRB PUBLICATION|Exercise D.|67 VideosQUANTUM NUMBERS AND GENERAL CHEMISTRY
GRB PUBLICATION|Exercise subjective type|34 VideosPRACTICAL ORGANIC CHEMISTRY
GRB PUBLICATION|Exercise Exercise 4 (Matrix match type )|1 VideosREDOX REACTIONS
GRB PUBLICATION|Exercise All Questions|332 Videos
Similar Questions
Explore conceptually related problems
GRB PUBLICATION-QUANTUM NUMBERS AND GENERAL CHEMISTRY-B.
- For nitrogen ataom ,if 5^(th) electron has quntum numbers n=2 ,l=1m=-1...
Text Solution
|
- which of the following quantum will have higher numercal value among r...
Text Solution
|
- pair of orbitals which have identical orientation and common nodal pl...
Text Solution
|
- Choose the correct option for the quantum numbers of the last electron...
Text Solution
|
- Select set of quantum numbers which is possible for maximum number of...
Text Solution
|
- Which is a possible set of quantum numbers for the unpaired electrons ...
Text Solution
|
- Principal azimuthal , and magnetic quantum numbers are respetively r...
Text Solution
|
- Degensrate atomic orbitals have :
Text Solution
|
- How many maximum electrons can be dessribed by the quantum number n=5...
Text Solution
|
- Any p- orbital can accomedate upto:
Text Solution
|
- which type of orbital is designated by n=2,l=3,m(1)=-2?
Text Solution
|
- which of the following statements regarding subdhell filling for s neu...
Text Solution
|
- Out of the following orbitals which have nodal plane perpendiclar to t...
Text Solution
|
- total number of possible unique sts of gour quantum numbers in the 5th...
Text Solution
|
- Which combinations of quantum number n,l,m,s for the electron in an a...
Text Solution
|
- Maximum number of orbitals are present for which of the following set ...
Text Solution
|
- m=- 1 is not possible for :
Text Solution
|
- the highest probability of finding the electron in an orbital having v...
Text Solution
|
- the maximum number of electrons that can be accommodated in the M^(th)...
Text Solution
|
- If an elevtronsd has spin quantum number of =(1)/(2) and magnetic qua...
Text Solution
|