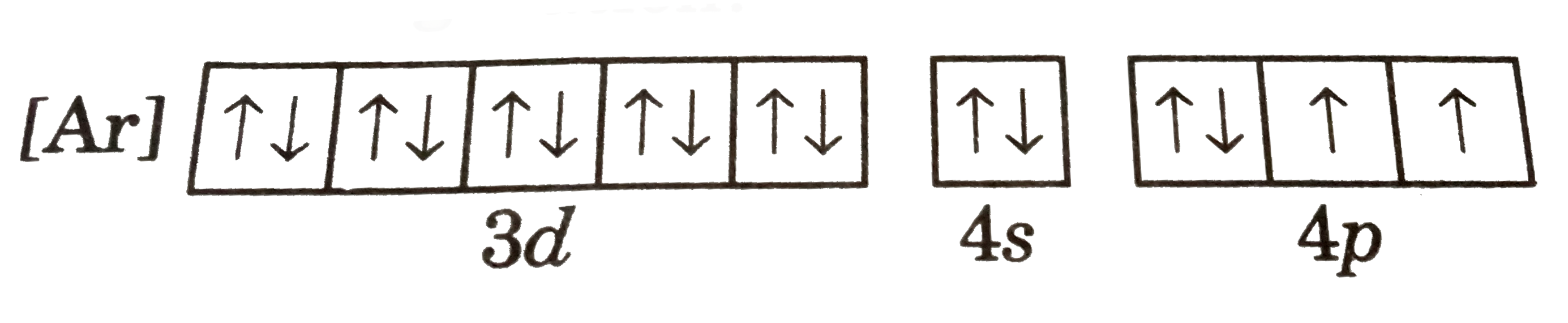
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
QUANTUM NUMBERS AND GENERAL CHEMISTRY
GRB PUBLICATION|Exercise E.|32 VideosQUANTUM NUMBERS AND GENERAL CHEMISTRY
GRB PUBLICATION|Exercise F.|15 VideosQUANTUM NUMBERS AND GENERAL CHEMISTRY
GRB PUBLICATION|Exercise C,|6 VideosPRACTICAL ORGANIC CHEMISTRY
GRB PUBLICATION|Exercise Exercise 4 (Matrix match type )|1 VideosREDOX REACTIONS
GRB PUBLICATION|Exercise All Questions|332 Videos
Similar Questions
Explore conceptually related problems
GRB PUBLICATION-QUANTUM NUMBERS AND GENERAL CHEMISTRY-D.
- The maximum no . Electrons in phosphorous atom for which n+l+m =3 will...
Text Solution
|
- If n = 6, the correct sequence for filling of electrons will be.
Text Solution
|
- Which element has the following ground state electrons configuration ?
Text Solution
|
- Hund's rule states that the most stable arrangement of electrons : (fo...
Text Solution
|
- the pauli exclusion principle states that :
Text Solution
|
- which of the following is correct set of quantum numbers for the last...
Text Solution
|
- Among the following , the configuration 1s^(2) 2s^(2)2p^(6)3s^(2)3p^(...
Text Solution
|
- which of the following electrons configuraation violate Hund's rule fo...
Text Solution
|
- The first excited state of Cl^(-) will have degenergy of :
Text Solution
|
- the percentage of orbitals occupied by electrons out to total orbitals...
Text Solution
|
- which of the following is vioation of (n+1) rule ?
Text Solution
|
- in which of the following change both the electrons are removed from s...
Text Solution
|
- The explanation for the presence of three unpaired electrons in the ni...
Text Solution
|
- The number of d-electron retained in Fe^(2) ("At no. of" Fe =26) ion ...
Text Solution
|
- in a muti- electron atom ,which of the following orbitals described n...
Text Solution
|
- Which of the following set of quantum numbers represents the highest e...
Text Solution
|
- The correct set of quantum number for the unpaired electron of chlorin...
Text Solution
|
- After np orbitals are filled, the next orbital filled will be :- (a)...
Text Solution
|
- Total number of electrons having n + l = 3 in Cr(24) atom in its groun...
Text Solution
|
- The possible value of l and m for the last electron in the Cl^(- )ion ...
Text Solution
|