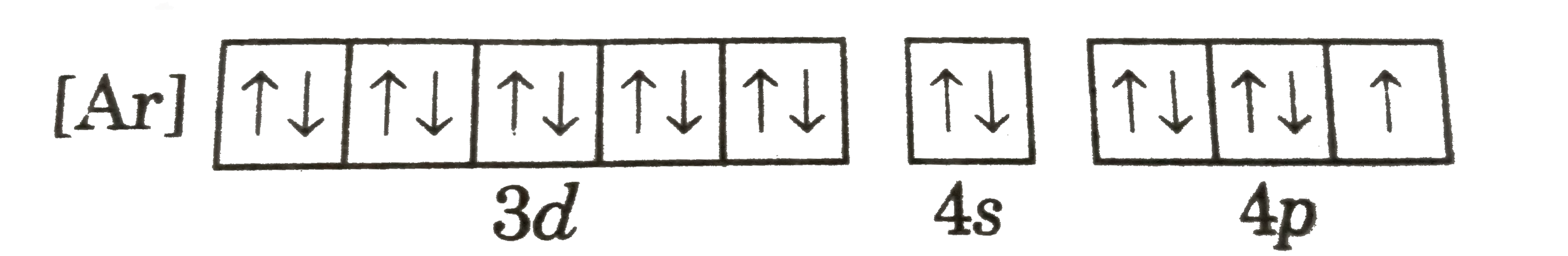
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
QUANTUM NUMBERS AND GENERAL CHEMISTRY
GRB PUBLICATION|Exercise E.|32 VideosQUANTUM NUMBERS AND GENERAL CHEMISTRY
GRB PUBLICATION|Exercise F.|15 VideosQUANTUM NUMBERS AND GENERAL CHEMISTRY
GRB PUBLICATION|Exercise C,|6 VideosPRACTICAL ORGANIC CHEMISTRY
GRB PUBLICATION|Exercise Exercise 4 (Matrix match type )|1 VideosREDOX REACTIONS
GRB PUBLICATION|Exercise All Questions|332 Videos
Similar Questions
Explore conceptually related problems
GRB PUBLICATION-QUANTUM NUMBERS AND GENERAL CHEMISTRY-D.
- Hund's rule of maximum spin multiplcity is not application for :
Text Solution
|
- Aufbau priciple does not give correct arrangmen of filling up of atom ...
Text Solution
|
- Which of the following sequeous is correct as pe Aufbau principle ?
Text Solution
|
- Find configuraation which does not follow Hund's rule of maximum multi...
Text Solution
|
- If spin quantum number has 4 values instead of two then identify the i...
Text Solution
|
- Assuming hund's Rule is not necesssarily follwed for a d^(5) configura...
Text Solution
|
- In ground state of phoosphorous atom (Z=15) , the numbers occupied su...
Text Solution
|
- which among th efollowing species has the same number o felectrons in ...
Text Solution
|
- which of the following is worng outer electonic configuration ?
Text Solution
|
- What is a possible set of quantum numbers for the unpired eletron in t...
Text Solution
|
- The quantum number of four electrons (el to e4) are given below :- ...
Text Solution
|
- If nitrogen atoms had el,ectonic configuration is ? It would have en...
Text Solution
|
- The quantum number , n=1,l=1,m(1)=0 could represent a valence electron...
Text Solution
|
- A gas phase atom with the electronic configyartin 1s^(2)2s^(2)2p^(6)...
Text Solution
|
- A sulphur atom in its ground state has the electrons confiuration 1...
Text Solution
|
- whcih of the following does NOT repesent the arrangement of electrons ...
Text Solution
|
- A M^(+2) ion derved fromn a metal in the first transition metal serie...
Text Solution
|
- Total number of electrons in Cu atom havijng m(1)=0 :
Text Solution
|
- which of the following stateement is coorect ?
Text Solution
|
- which of the following cation has only two eclctrons In its last shell...
Text Solution
|