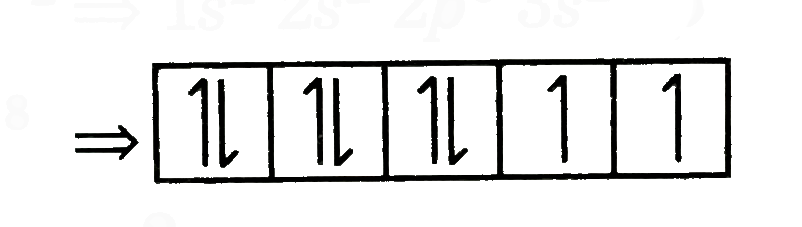
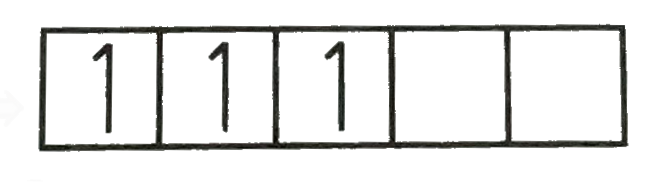
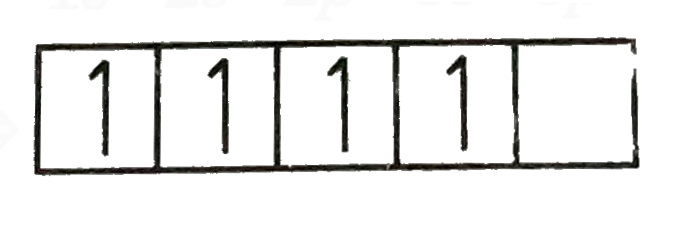A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
QUANTUM NUMBERS AND GENERAL CHEMISTRY
GRB PUBLICATION|Exercise F.|15 VideosQUANTUM NUMBERS AND GENERAL CHEMISTRY
GRB PUBLICATION|Exercise reasoning type|6 VideosQUANTUM NUMBERS AND GENERAL CHEMISTRY
GRB PUBLICATION|Exercise D.|67 VideosPRACTICAL ORGANIC CHEMISTRY
GRB PUBLICATION|Exercise Exercise 4 (Matrix match type )|1 VideosREDOX REACTIONS
GRB PUBLICATION|Exercise All Questions|332 Videos
Similar Questions
Explore conceptually related problems
GRB PUBLICATION-QUANTUM NUMBERS AND GENERAL CHEMISTRY-E.
- How many unpaired electrons are present in a ground state?
Text Solution
|
- How many unpaired electrons are there in Ni^(2+)?
Text Solution
|
- Identify the cation having maximum magnetic moment :
Text Solution
|
- Which of the following have maximum number of unpaired electrons ?
Text Solution
|
- Ions which have maximum total spin muliplicity ?
Text Solution
|
- In which of the following ,the two species have different value of spi...
Text Solution
|
- In which of the following set of species have same magnetic nature a...
Text Solution
|
- Which of the following has maximum number of unpaired electron (atomic...
Text Solution
|
- An ion (Mn^(a+)) has the magnetic moment equal to 4.9 B.M What is the ...
Text Solution
|
- The spin-only magnetic moment [in units of Bohr magneton, (mu(B) of Ni...
Text Solution
|
- which of the following ions has the maximum magnetic moment ?
Text Solution
|
- The value of the magnetic moment of a particular ion is 2.83 Bohr magn...
Text Solution
|
- What is the total spin value in case of (26)Fe^(3+) ion ?
Text Solution
|
- Magnetic moment of Fe^(a+)(Z= 26 ) is sqrt 24 BM. Hence number of unpa...
Text Solution
|
- Which of the following ions has the maximum number of unpaired d- ele...
Text Solution
|
- The total spin resulting from a d^7 configuration is :
Text Solution
|
- Which of the following having same value of magnetic moment?
Text Solution
|
- which of the following species are paramagnetic I nature ?
Text Solution
|
- Calcualate the spin multiplicity for H-atom .
Text Solution
|
- Magnetic moment of (30)Zn^(2+) ion is same as :
Text Solution
|