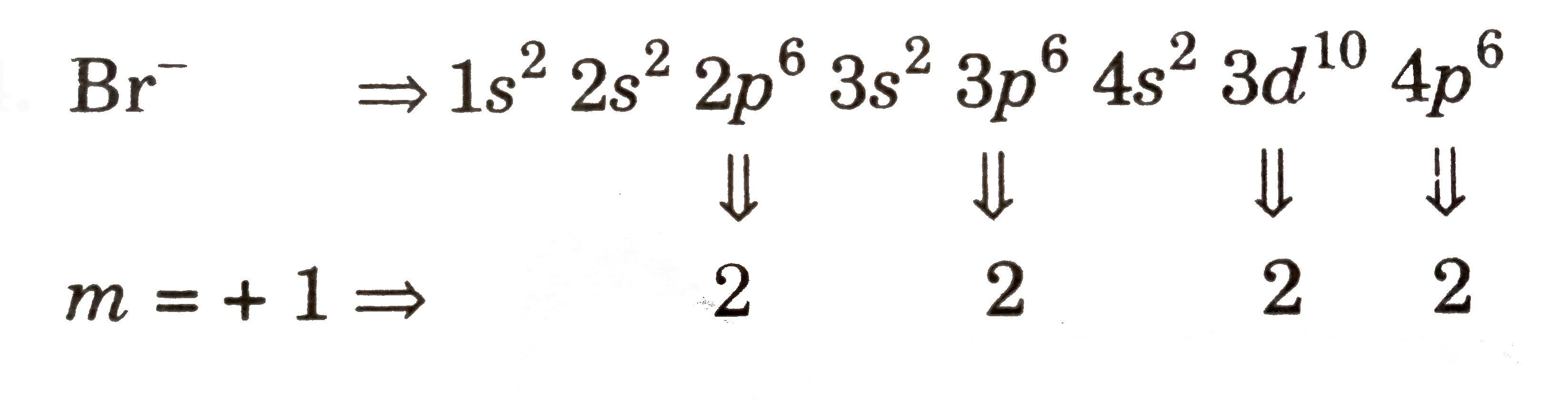Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
GRB PUBLICATION-QUANTUM NUMBERS AND GENERAL CHEMISTRY-subjective type
- An electrons resdes in a subshell which has 7 number associated wi...
Text Solution
|
- Calculate the total number of electrons for Mn having n+l+m=2
Text Solution
|
- Find the number of electrons are there in NI^(2+) ion which are h...
Text Solution
|
- How many unpaired electrons are there in Ni^(2+)?
Text Solution
|
- the maximum number of electrons that can be accommodarted in all t...
Text Solution
|
- in species X^(2+) , the mass number is 20 and number of neutrons ...
Text Solution
|
- the magnetic moment value of species X is 4.48 B.M fnd out the num...
Text Solution
|
- Find the difference between Z("eff) of 4s and 3d electrons of Sc.
Text Solution
|
- Calculate the total number of p-orbitals electrons present in Ag (47) ...
Text Solution
|
- The mass number of element 'X' is 'A' . If X^(4-) contains 10 electron...
Text Solution
|
- Calcualate the value of Z("eff") on 3d electrons of Sc .
Text Solution
|
- calculate the difference between th Z("eff") exerenced by ouuterm...
Text Solution
|
- for a hydrothertical atom find out the total number of electrons...
Text Solution
|
- Find the number of electrons in Pd having nxxl =0and |m|gt1
Text Solution
|
- (p) if azimythal quantum number value is 3, the maximum value of ...
Text Solution
|
- n+l +m for the valence electrons of rb will be (where n,l,m, are ...
Text Solution
|
- If aufbau's rule is not followed and electrons foilling if done ...
Text Solution
|
- Maximum number of electrons having quantum numbers n=5,|m(1)|=2...
Text Solution
|
- The first excited state of Cl^(-) will have the energy of :
Text Solution
|
- the number of species which are dimagnetic amongest the following : ...
Text Solution
|