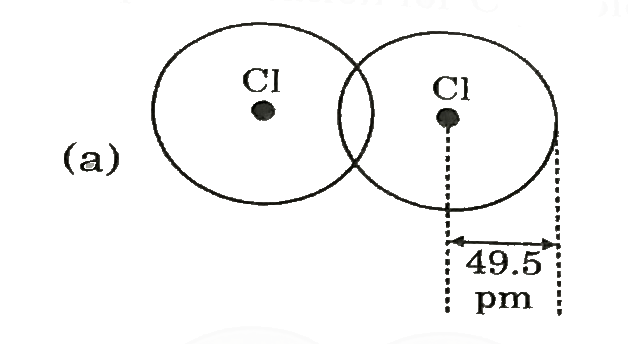
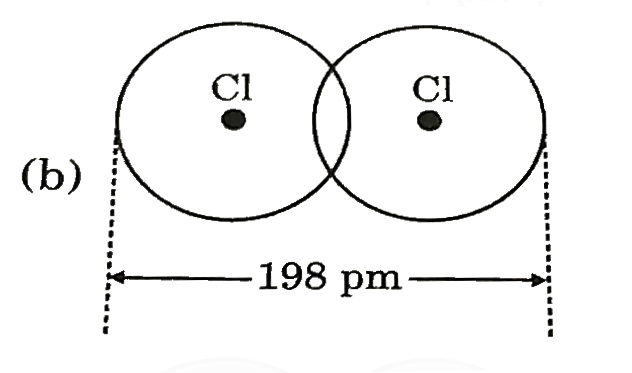
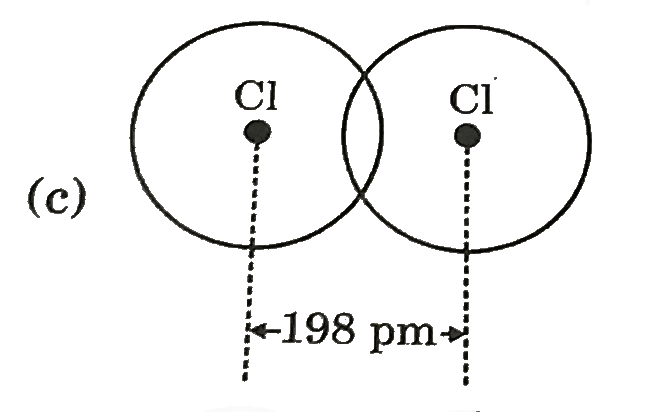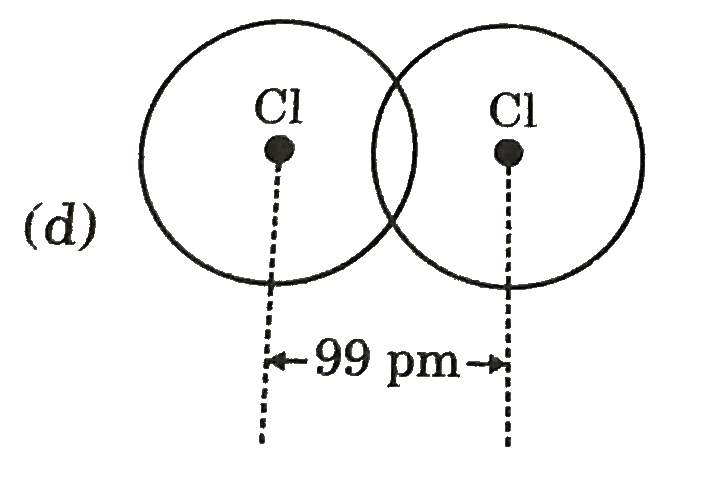A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
PERIODIC TABLE
GRB PUBLICATION|Exercise C.Ionisation Energy|93 VideosPERIODIC TABLE
GRB PUBLICATION|Exercise D. Electron Gain Enthalpy (Electron Affinity)|50 VideosPERIODIC TABLE
GRB PUBLICATION|Exercise Subjective Type|32 VideosNOMENCLATURE AND CLASSIFICATION
GRB PUBLICATION|Exercise Subjective Type|24 VideosPRACTICAL ORGANIC CHEMISTRY
GRB PUBLICATION|Exercise Exercise 4 (Matrix match type )|1 Videos
Similar Questions
Explore conceptually related problems
GRB PUBLICATION-PERIODIC TABLE-B.Atomic and Ionic Radius
- When the atoms: Ba,Cs,Mg,Na are arranged in order of increasing size, ...
Text Solution
|
- Which element has the largest atomic radius?
Text Solution
|
- When the atoms, P(Z = 15), S (Z = 16) and As(Z = 33), are arranged in ...
Text Solution
|
- Which property decreases from left to right across the periodic table ...
Text Solution
|
- Consider the ions Li^(+),Na^(+),Be^(2+) and Mg^(2+). Which two are clo...
Text Solution
|
- Which pair of elements have chemical propeties that are the most simil...
Text Solution
|
- When the atoms Li,Be,B and Na are arranged in order of increasing atom...
Text Solution
|
- Which property of an element is most dependent on this shielding effec...
Text Solution
|
- Which element has the largest atomic radius?
Text Solution
|
- The atomic radius of elements of which of the following series would b...
Text Solution
|
- The radii of F,F^(-),O and O^(-2) are in the order of
Text Solution
|
- Which of the following case the size ratio is minimum?
Text Solution
|
- For the element X, student Surbhi measured its radius as 102 nm, Mr. G...
Text Solution
|
- The species having smallest ionic radius is:
Text Solution
|
- In the crystals of which of the following ionic compounds would you ex...
Text Solution
|
- CONSEQUENE OF LANTHANIDE CONTRACTION
Text Solution
|
- Which of the following have total 18 electrons and it is larger than C...
Text Solution
|
- The size of isoelectronic species F^(ɵ), Ne, and Na^(+) affected by
Text Solution
|
- Which of the following order of ionic-radius is correct?
Text Solution
|
- Covalent radius of Ci si 99 pm. Select best representation for CI(2) m...
Text Solution
|