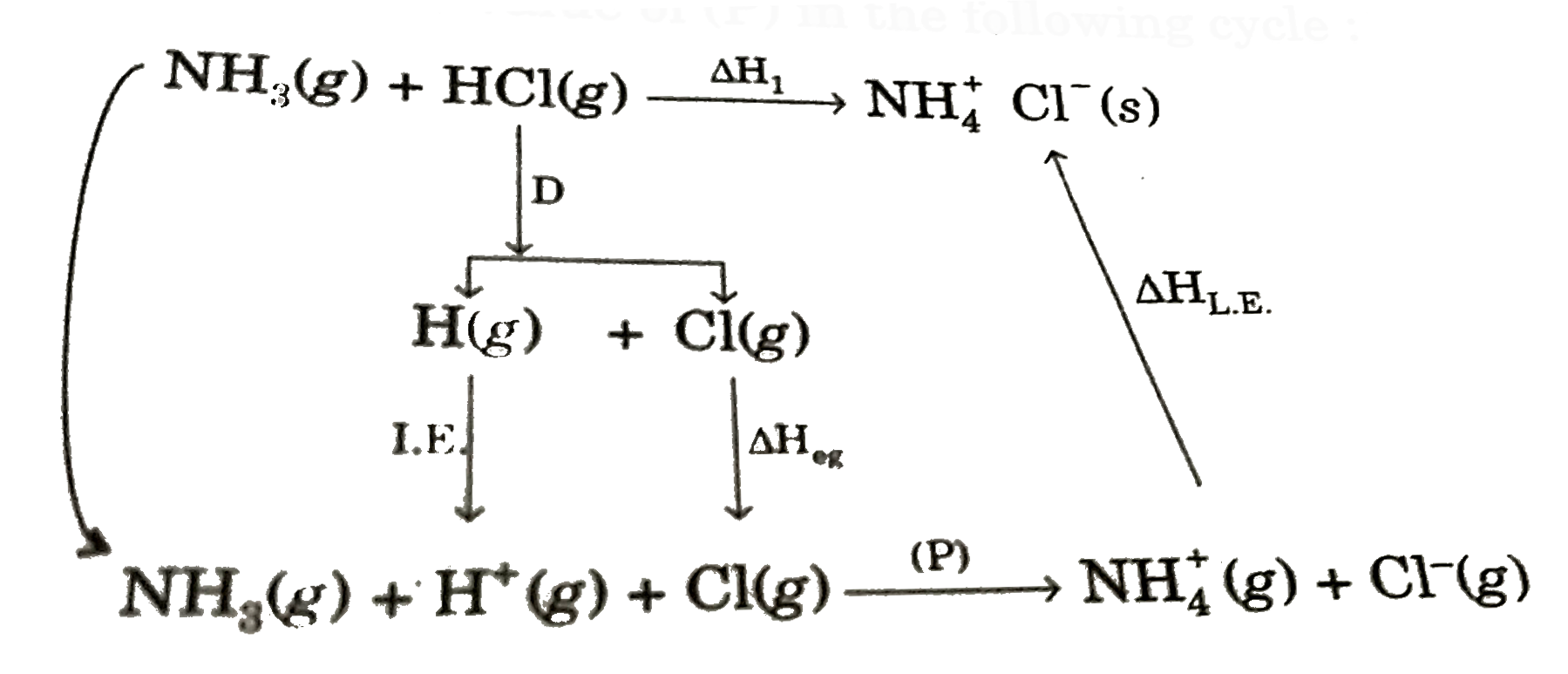A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
PERIODIC TABLE
GRB PUBLICATION|Exercise Reasoning Type|29 VideosPERIODIC TABLE
GRB PUBLICATION|Exercise Multiple Objective Type|64 VideosPERIODIC TABLE
GRB PUBLICATION|Exercise E.Electronegativity|54 VideosNOMENCLATURE AND CLASSIFICATION
GRB PUBLICATION|Exercise Subjective Type|24 VideosPRACTICAL ORGANIC CHEMISTRY
GRB PUBLICATION|Exercise Exercise 4 (Matrix match type )|1 Videos
Similar Questions
Explore conceptually related problems
GRB PUBLICATION-PERIODIC TABLE-F.Acidic-Basic Character, Hydration etc.
- Which oxide produces the most acidic solution when 0.1 mol is added to...
Text Solution
|
- Element P,Q, R and S belong to the same group. The oxide of P is acid...
Text Solution
|
- Increasing order of basic character of NO(2)K(2)O and ZnO is
Text Solution
|
- Which of the following options is correct regarding periodic propertie...
Text Solution
|
- Condition suitable for high solubility of ionic compound in solvent sh...
Text Solution
|
- Find out the value of (P) in the following cycle: Given DeltaH(1...
Text Solution
|
- Which terms are exothermic for the formation of NaF(s)? (P) Na(g) ra...
Text Solution
|
- In order to calculate the lattice energy of NaCI using a Born-Haber cy...
Text Solution
|
- Which of the following represent correct of hydration energy released ...
Text Solution
|
- The following order are given along with their metioned properties: ...
Text Solution
|
- Which of the following order is/are correct ?
Text Solution
|
- The following acid have arrange in the order of decreasing strength. I...
Text Solution
|
- Which of the following is/are correct statement(s)?
Text Solution
|
- Which of the following is an amphoteric oxide?
Text Solution
|
- Which is an amphoteric oxides?
Text Solution
|
- The order of basic strength of given oxide:
Text Solution
|
- Which of the following is neutral oxide?
Text Solution
|
- The high oxidising power of fluorine is due to :
Text Solution
|
- The correct statement is:
Text Solution
|
- A,B and C are oxides of elements X,Y and Z respectively. X,Y and Z are...
Text Solution
|