A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
PERIODIC TABLE
GRB PUBLICATION|Exercise Comprehension 2|1 VideosPERIODIC TABLE
GRB PUBLICATION|Exercise Comprehension 3|1 VideosPERIODIC TABLE
GRB PUBLICATION|Exercise Comprehension 1|1 VideosNOMENCLATURE AND CLASSIFICATION
GRB PUBLICATION|Exercise Subjective Type|24 VideosPRACTICAL ORGANIC CHEMISTRY
GRB PUBLICATION|Exercise Exercise 4 (Matrix match type )|1 Videos
Similar Questions
Explore conceptually related problems
GRB PUBLICATION-PERIODIC TABLE-Comprehension
- In the modern periodic table, elements are arranged in order of increa...
Text Solution
|
- Ionisation energies three hypothetical elements are given below (in kJ...
Text Solution
|
- Ionisation energies three hypothetical elements are given below (in kJ...
Text Solution
|
- Ionisation energies three hypothetical elements are given below (in kJ...
Text Solution
|
- In 1931, Pauling defined the electronegativity of an atom as the tende...
Text Solution
|
- In 1931, Pauling defined the electronegativity of an atom as the tende...
Text Solution
|
- In above graph element F is:
Text Solution
|
- The amount of energy released when an electron is added to an isolated...
Text Solution
|
- The amount of energy released when an electron is added to an isolated...
Text Solution
|
- The amount of energy released when an electron is added to an isolated...
Text Solution
|
- The properties of the elements (atomic/ionic radii, electron gain enth...
Text Solution
|
- The properties of the elements (atomic/ionic radii, electron gain enth...
Text Solution
|
- The properties of the elements (atomic/ionic radii, electron gain enth...
Text Solution
|
- The properties of the elements (atomic/ionic radii, electron gain enth...
Text Solution
|
- A quantitative measure of the tendency of an element to lose electron ...
Text Solution
|
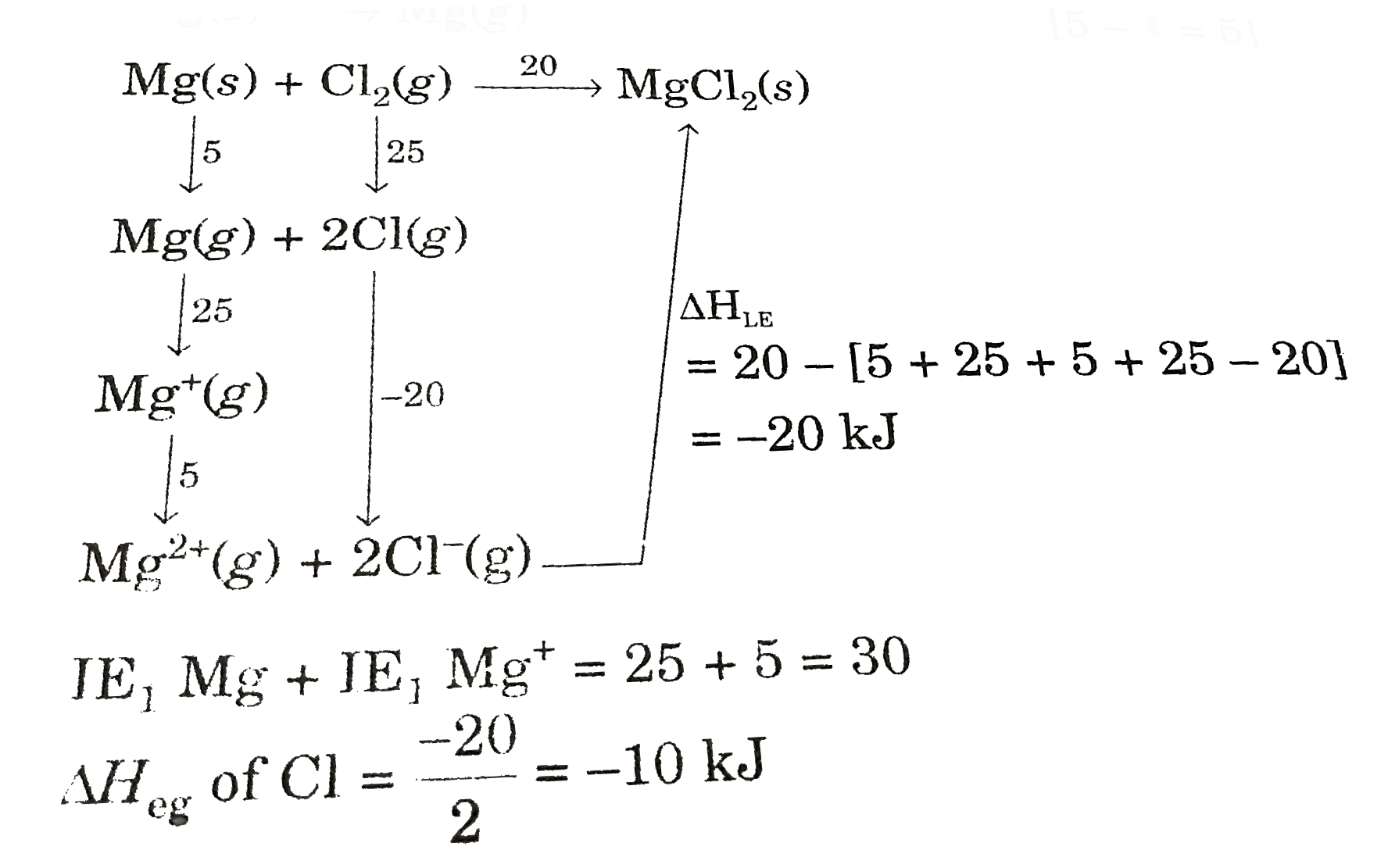
- Born Haber cycle helps to determine lattice enthalpy of ionic compound...
Text Solution
|
- Born Haber cycle helps to determine lattice enthalpy of ionic compound...
Text Solution
|
- Nitrogen can form many oxides with oxygen, and thus is said to exhibit...
Text Solution
|
- The size of any species depends on various factors such as nature of c...
Text Solution
|
- Ionisation energies of unknown elements are given below : |{:("Eleme...
Text Solution
|