Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
PERIODIC TABLE
GRB PUBLICATION|Exercise Subjective Type|32 VideosPERIODIC TABLE
GRB PUBLICATION|Exercise Comprehension 20|1 VideosNOMENCLATURE AND CLASSIFICATION
GRB PUBLICATION|Exercise Subjective Type|24 VideosPRACTICAL ORGANIC CHEMISTRY
GRB PUBLICATION|Exercise Exercise 4 (Matrix match type )|1 Videos
Similar Questions
Explore conceptually related problems
GRB PUBLICATION-PERIODIC TABLE-Match The Column Type
- Match the following columns
Text Solution
|
- Match the Column-I to Column-II :
Text Solution
|
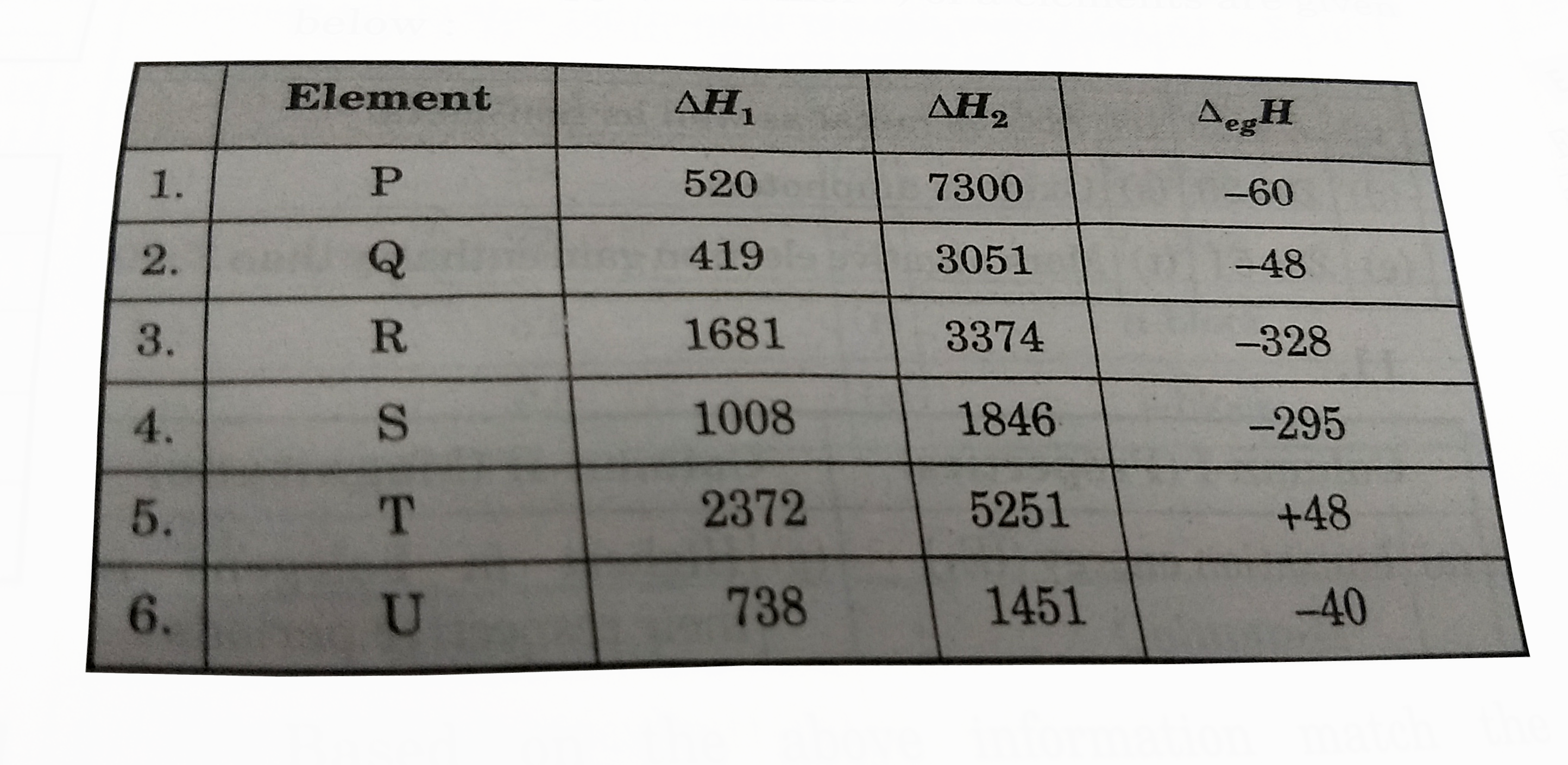
- The first (DeltaH(1)) and second (DeltaH(2)) ionisation enthalpies (in...
Text Solution
|
- Match the following columns
Text Solution
|
- Column-I contains some increasing orders of various species and column...
Text Solution
|
- Column-I contains the atomic number of few elements and column-II has ...
Text Solution
|
- Match the following columns
Text Solution
|
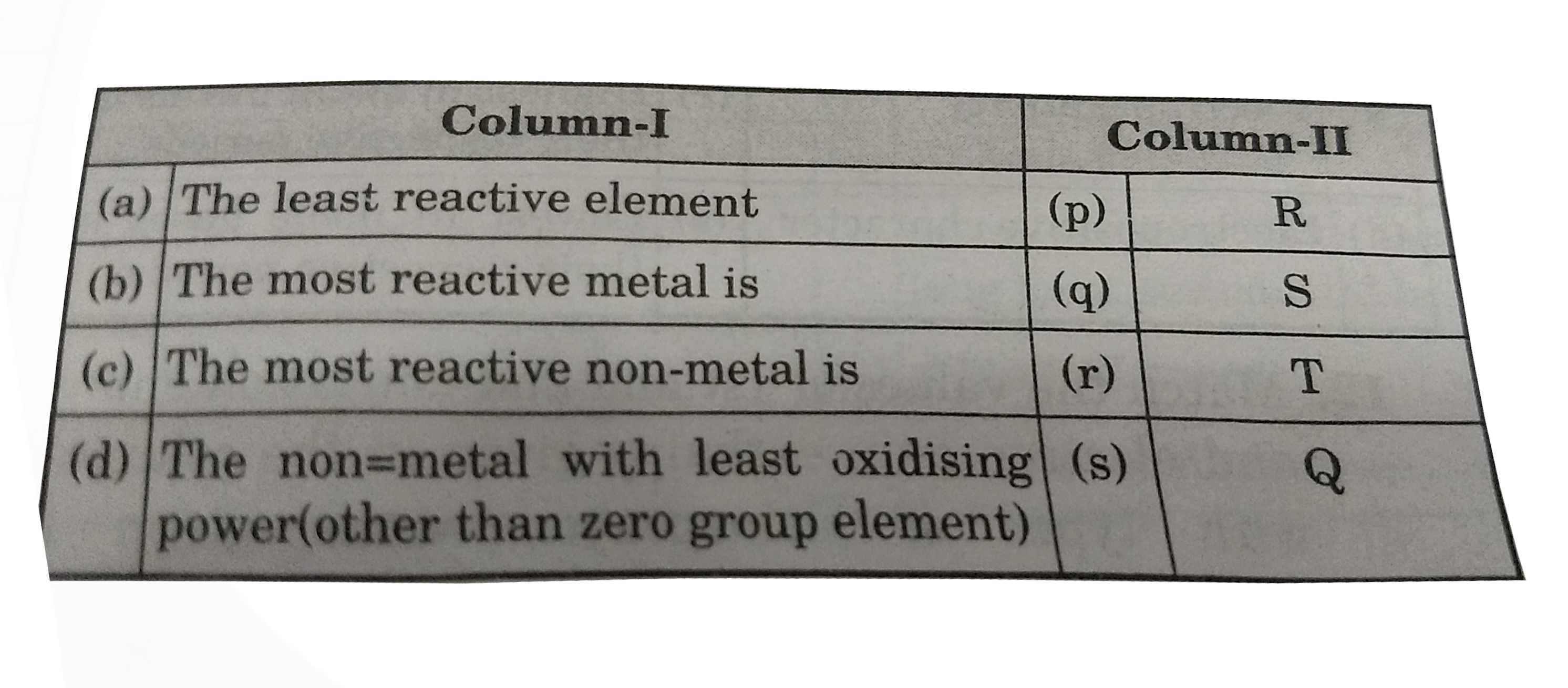
- Match the values of 1st and 2nd ionisation energy and electron gain en...
Text Solution
|
- Match the following columns
Text Solution
|
- Match the following columns
Text Solution
|
- Match the following columns
Text Solution
|
- Match the following Columns
Text Solution
|
Text Solution
|
- Match the following columns
Text Solution
|
- Match the correct ionisation enthalpies electron gain enthalpies of th...
Text Solution
|
- Match the following columns
Text Solution
|
- Match the following columns
Text Solution
|
- Match the order of species in column-I with properties in column-II.
Text Solution
|
- Match the following columns
Text Solution
|
- Match column-II (atomic number of elements) with column-II (position o...
Text Solution
|