Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
GRB PUBLICATION-PERIODIC TABLE-Subjective Type
- 0.5 moles of gaseous non-metallic X^(-) anions (having positive elect...
Text Solution
|
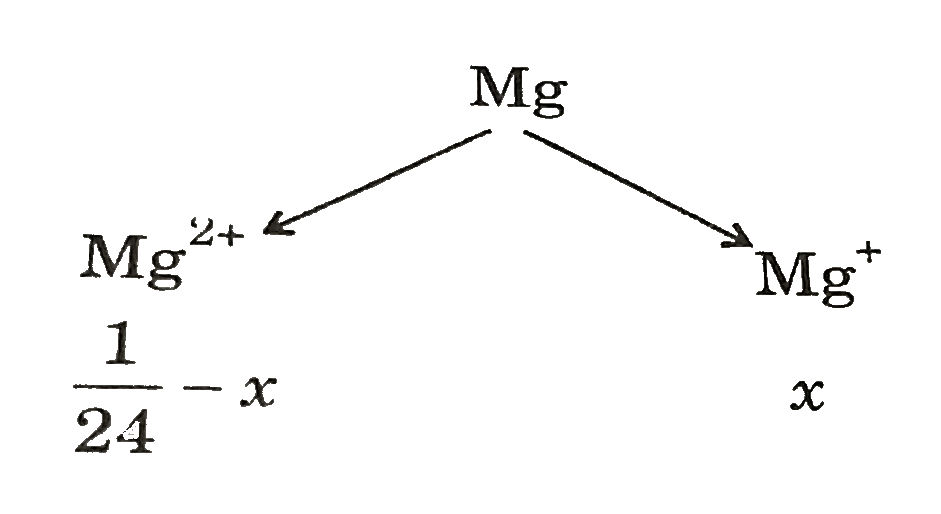
- 1.0 g of Mg atom ("atomic mass" = 24.0 "amu") in the vapour phase abso...
Text Solution
|
- Find the sum of total number of 5f-electrons in Th and Ac.
Text Solution
|
- Find the total number of species having two unpaired electrons from th...
Text Solution
|
- If internuclear distance between A atoms in A(2) is 10Å and between B ...
Text Solution
|
- Find the number of p-block elements from the following atomic numbers ...
Text Solution
|
- Find the total number of 6^(th) period elements from the given atomic ...
Text Solution
|
- Find the total number of paramagnetic species among the following? S...
Text Solution
|
- What is the group number of Ba in periodic table?
Text Solution
|
- Select the number of elements which are called transition metals. B,...
Text Solution
|
- Among the following species, how many have their ionic size greater th...
Text Solution
|
- Among the following find out the total number of d-block elements. L...
Text Solution
|
- Number of oxides which is/are more basic as compared to Na(2)O. Li(2...
Text Solution
|
- Bond length of A-A bond is 124 pm and bond length of B-B bond is 174 p...
Text Solution
|
- Find the number of chemical species in which outer shell 'd' orbital i...
Text Solution
|
- Find the number of transition elements in the following: Zn, Cd,Hg, ...
Text Solution
|
- According to Hannay-Smith formula, if E.N difference between A and B i...
Text Solution
|
- The element with the lowest atomic number that has a ground-state elec...
Text Solution
|
- Find the total number of correct orders among the following. (a) Ord...
Text Solution
|
- Period number of Sc = x Modern periodic table group number of TI = x...
Text Solution
|