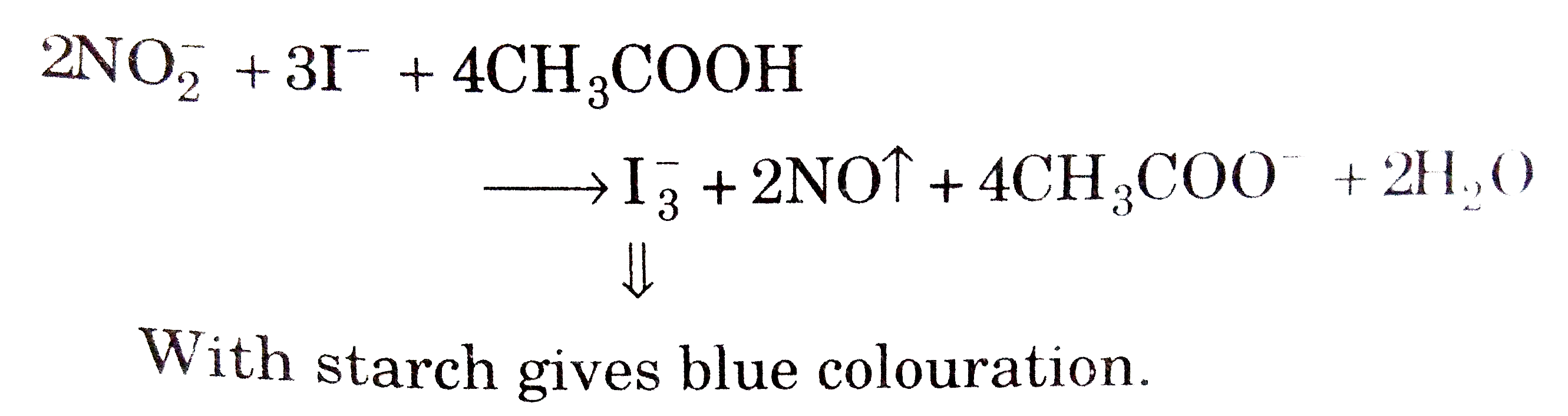A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
SALT ANALYSIS
GRB PUBLICATION|Exercise D.Class-A Subgroup-II Acidic Radicals|39 VideosSALT ANALYSIS
GRB PUBLICATION|Exercise E.Precipitation/Redox Reacttions|48 VideosSALT ANALYSIS
GRB PUBLICATION|Exercise B. Dry Tests/Colour/Solubility/Heating Effects|59 VideosS BLOCK ELEMENTS
GRB PUBLICATION|Exercise SUBJECTIVE TYPE|9 VideosTHERMODYNAMICS
GRB PUBLICATION|Exercise All Questions|704 Videos
Similar Questions
Explore conceptually related problems
GRB PUBLICATION-SALT ANALYSIS-C.Class- A Subgroup-I-Acidic Radicals
- A sodium salt on treatment with MgCl(2) gives white precipitate only o...
Text Solution
|
- When a salt is heated with dilute H(2)SO(4) " and " KMnO(4) solution, ...
Text Solution
|
- Solution of a salt in dilute H(2)SO(4) or acetic and produces deep blu...
Text Solution
|
- Which of the following compound(s) slowly disappears on prologed passa...
Text Solution
|
- (P) Salt of X + CaCI(2) (excess) rarr Y + Filtrate (Q) Filtrate +NH(3)...
Text Solution
|
- A and B are:
Text Solution
|
- Which of the following options is correct? (P) HgCI(2) can be used f...
Text Solution
|
- Which of the following produced colourless gas with dil. H(2)SO(4)?
Text Solution
|
- Which of the following pair of salts produces gas lacking pungent odou...
Text Solution
|
- Which of the following salts will not produce any observable changes w...
Text Solution
|
- Which of the following pair of compounds cannot co-exist in aqueous so...
Text Solution
|
- CO(3)^(2-) and SO(3)^(2-) cannot be distinguished by:
Text Solution
|
- CO(3)^(2-) and HCO(3)^(-) can be distingusihed by:
Text Solution
|
- A compound X on heating gives a colourless gas. This residue is dissol...
Text Solution
|
- The co-ordination number of central ion of the complex obtained in the...
Text Solution
|
- CO(3)^(2-)+2HCI (dil.) rarr Gas + Solution Resulting gas produces salt...
Text Solution
|
- When 6 M hydrochloric acid is added to an unknonw white solid, a colou...
Text Solution
|
- Which reagents produce a gas when combined? (P) HCI and Na(2)SO(3) ...
Text Solution
|
- Which gas turns lime water, a saturated solution of Ca(OH)(2) cloudy?
Text Solution
|
- Salt X + dil. H(2)SO(4) rarr(B) +H(2)O Salt Y + conc. H(2)SO(4) rarr...
Text Solution
|