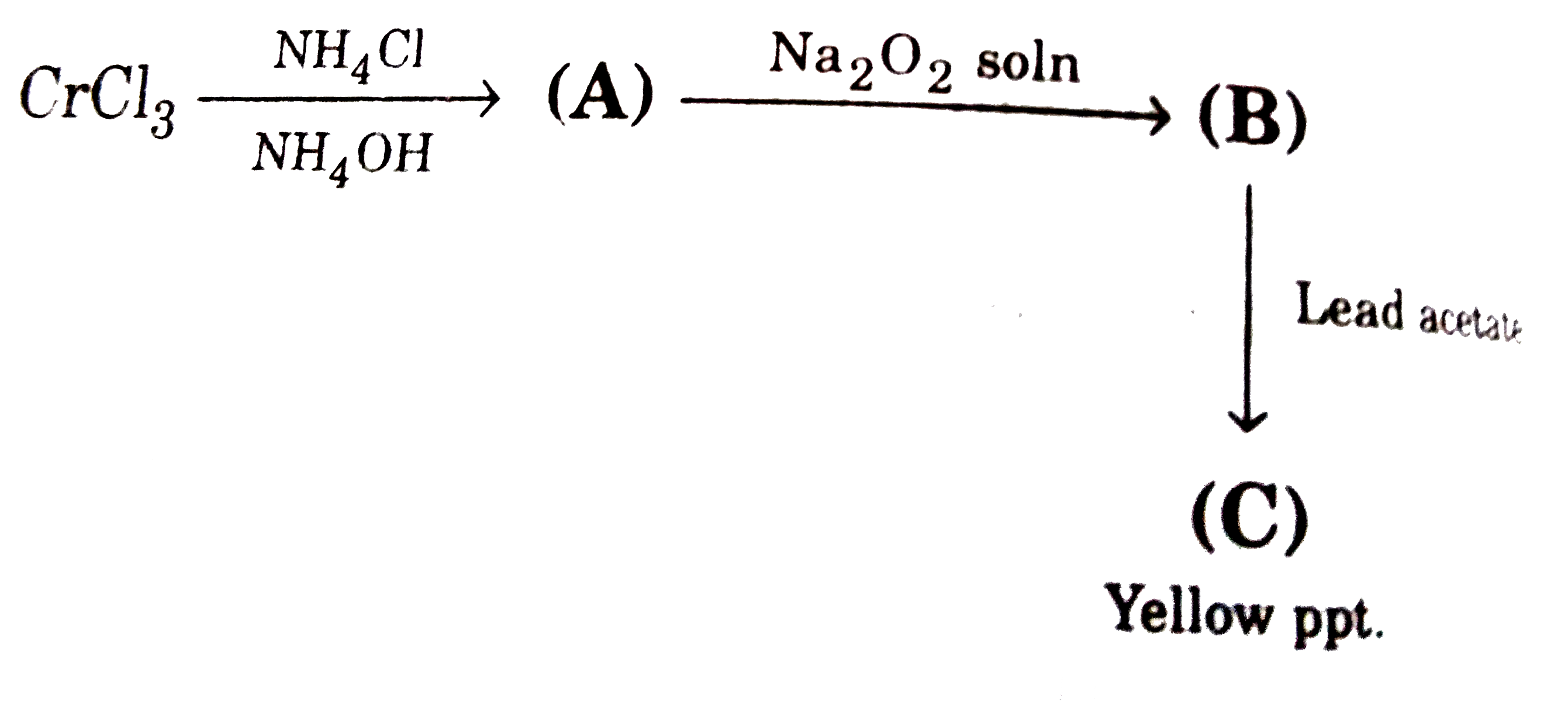A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
SALT ANALYSIS
GRB PUBLICATION|Exercise J: IVth Group Cations|24 VideosSALT ANALYSIS
GRB PUBLICATION|Exercise K: Vth and Vth Group Cations|10 VideosSALT ANALYSIS
GRB PUBLICATION|Exercise H: IInd Group Cations|49 VideosS BLOCK ELEMENTS
GRB PUBLICATION|Exercise SUBJECTIVE TYPE|9 VideosTHERMODYNAMICS
GRB PUBLICATION|Exercise All Questions|704 Videos
Similar Questions
Explore conceptually related problems
GRB PUBLICATION-SALT ANALYSIS-I. IIIrd Group Cations
- {:(Fe(2)(SO(4))(3)+KCN(excess)),(" "darr),(underset((X))(K(3)[Fe(...
Text Solution
|
- Element A is d-block as well as transition element of 3d series it is ...
Text Solution
|
- Which d-orbital is not taking part in hybridisation of species B?
Text Solution
|
- Complex salt (X) gives....colour with AgNO(3). Complex (X) is formed b...
Text Solution
|
- The cation which gives white precipitate of its hydroxide when treated...
Text Solution
|
- Which of the following statement(s) is (are) incorrect?
Text Solution
|
- CrCI(3) solution +Na(2)S solution rarr ppt (A) The correct formula and...
Text Solution
|
- Which of the following is correct?
Text Solution
|
- Fe^(2+) does not give prussian blue colour with K(4)[Fe(CN)(6)] but on...
Text Solution
|
- When HNO(3) is added to sodium ferrocyanide, which of the following ob...
Text Solution
|
- What product is formed by mixing the solution of K(4)[Fe(CN)(6)] with ...
Text Solution
|
- Select the correct statement with respect to Fe^(3+) ions:
Text Solution
|
- Which one of the following compounds on reaction with Na(2)O(2) in alk...
Text Solution
|
- How do we differentiate between Fe^(3+) and Cr^(3+) in group III^(rd)?
Text Solution
|
- A mixture of chlorides of copper, cadmium, chromium, iron and aluminiu...
Text Solution
|
- CrO(4)^(2-)+H^(+) +H(2)O(2) overset("ether")rarr X +H(2)O Identify t...
Text Solution
|
- FeCI(3)+K(3)[Fe(CN)(6)] +H(2)O(2) overset(OH^(-))rarr Precipitate. The...
Text Solution
|
- CrCl(3) underset(NH(4)OH)overset(NH(4)Cl)to (A) underset(H(2)O)overset...
Text Solution
|
- Give the correct order of initials T or F for following statements. Us...
Text Solution
|
- Fe^(2+) and Fe^(3+) can be distinguished by
Text Solution
|