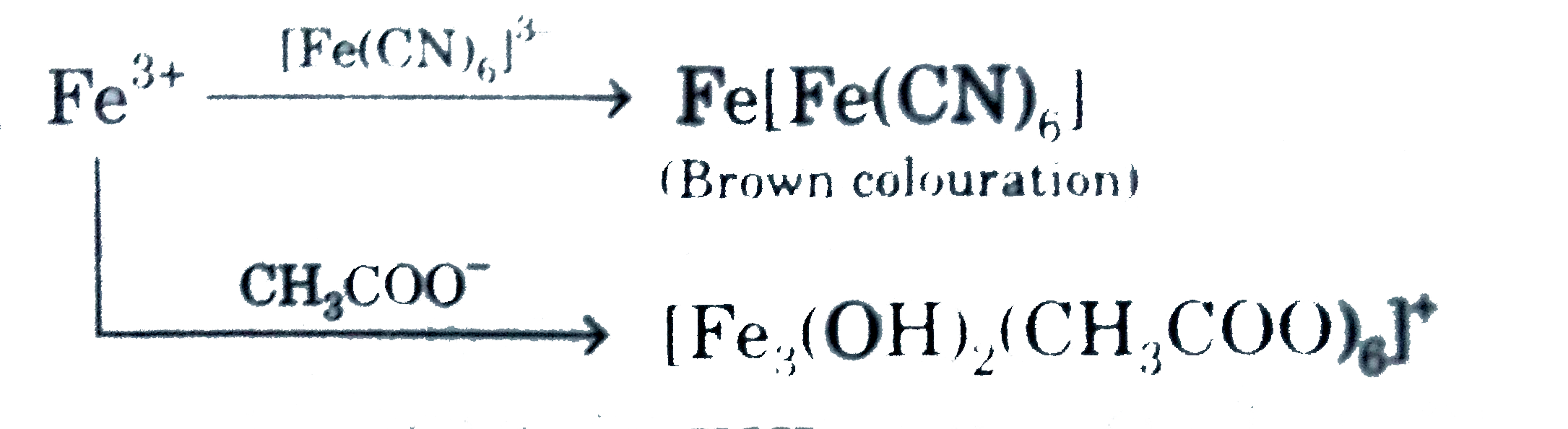A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
SALT ANALYSIS
GRB PUBLICATION|Exercise J: IVth Group Cations|24 VideosSALT ANALYSIS
GRB PUBLICATION|Exercise K: Vth and Vth Group Cations|10 VideosSALT ANALYSIS
GRB PUBLICATION|Exercise H: IInd Group Cations|49 VideosS BLOCK ELEMENTS
GRB PUBLICATION|Exercise SUBJECTIVE TYPE|9 VideosTHERMODYNAMICS
GRB PUBLICATION|Exercise All Questions|704 Videos
Similar Questions
Explore conceptually related problems
GRB PUBLICATION-SALT ANALYSIS-I. IIIrd Group Cations
- FeCI(3)+K(3)[Fe(CN)(6)] +H(2)O(2) overset(OH^(-))rarr Precipitate. The...
Text Solution
|
- CrCl(3) underset(NH(4)OH)overset(NH(4)Cl)to (A) underset(H(2)O)overset...
Text Solution
|
- Give the correct order of initials T or F for following statements. Us...
Text Solution
|
- Fe^(2+) and Fe^(3+) can be distinguished by
Text Solution
|
- An aqueous solution of a salt X turns blood red on treatment with SCN^...
Text Solution
|
- Ferric ion forms a prussian blue coloured ppt. due to
Text Solution
|
- Which of these ions is expected to be coloured in aqueous solution? ...
Text Solution
|
- Which 2.00M solution can be used to separate AI^(3+) from Fe^(3+) in a...
Text Solution
|
- Choose the reaction in which red colour precipitate/solution is not ob...
Text Solution
|
- A red colouration or precipitate is not obtained when:
Text Solution
|
- Which of the following precipitate(s) does /do not dissolve in excess ...
Text Solution
|
- When NH(4)Cl is added to a solution of NH(4)OH :
Text Solution
|
- The solution of sodium metal aluminate on diluting with water and then...
Text Solution
|
- An original solution of an inorganic salt in dilute HCl gives a brown ...
Text Solution
|
- Intense blue precipitate of Fe(4)[Fe(CN)(6)](3) and potassium hydroxid...
Text Solution
|
- Turnbull's blue is a .............
Text Solution
|
- Fe(OH)(3) and Cr(OH)(3) precipitates can be completely separated by :
Text Solution
|
- Ferric alum gives deep red colour with NH(4)SCN due to the formation o...
Text Solution
|
- NH(4)SCN can be used to test one or more out of Fe^(3+) , Co^(2+) , Cu...
Text Solution
|
- K(4)[Fe(CN)(6)] can be used to detect one or more out of Fe^(2+),Fe^(3...
Text Solution
|