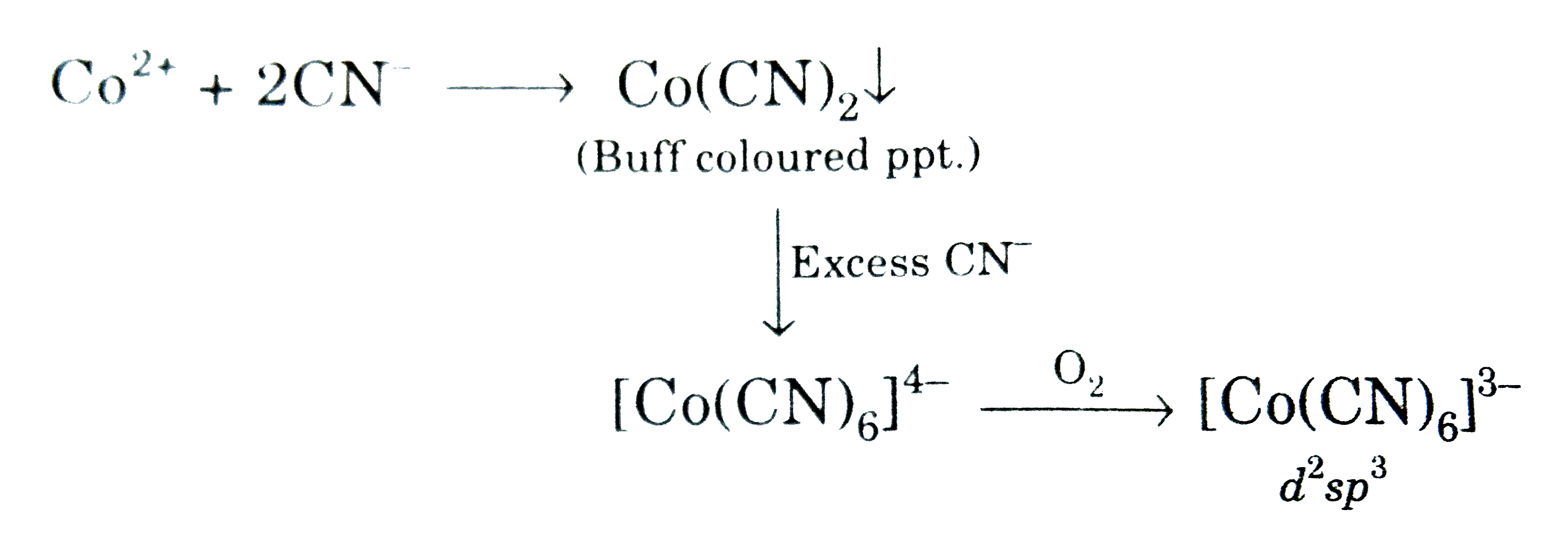A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
SALT ANALYSIS
GRB PUBLICATION|Exercise K: Vth and Vth Group Cations|10 VideosSALT ANALYSIS
GRB PUBLICATION|Exercise L: Miscellaneous|40 VideosSALT ANALYSIS
GRB PUBLICATION|Exercise I. IIIrd Group Cations|36 VideosS BLOCK ELEMENTS
GRB PUBLICATION|Exercise SUBJECTIVE TYPE|9 VideosTHERMODYNAMICS
GRB PUBLICATION|Exercise All Questions|704 Videos
Similar Questions
Explore conceptually related problems
GRB PUBLICATION-SALT ANALYSIS-J: IVth Group Cations
- An aqueous solution contains both Al^(3+) & Zn^(2+).To this solution N...
Text Solution
|
- A solution containing SCN^(-) ions can be used to test one or more out...
Text Solution
|
- The auxiliary reagent in group-IV reagent is:
Text Solution
|
- To avoid the precipitation of hydroxides of Ni^(2+),Co^(2+),Mn^(2+) al...
Text Solution
|
- A blue colouration (in solution or precipitate) is not obtained when:
Text Solution
|
- In the fourth group, white precipitate of Mn(OH)(2) on heating with Pb...
Text Solution
|
- A coloured solution of a salt gives following reactions: I. It gives...
Text Solution
|
- Zn(OH)(2)darr is soluble in:
Text Solution
|
- Which of the following metal sulphides has maximum solubility in water...
Text Solution
|
- A reddish pink substance on heating gives off a vapour which condenses...
Text Solution
|
- Among the following, buff colour metal sulphide is:
Text Solution
|
- Black colour compound A is insoluble in HCI solution but soluble in aq...
Text Solution
|
- When H(2)S gas a passed through an ammonical salt solution X, a slight...
Text Solution
|
- Buff coloured cyanide compound (A) reacts with excess of KCN in presen...
Text Solution
|
- Among the following, brownish black colour metal sulphide is:
Text Solution
|
- How many structural isomers can be possible for yellow complex?
Text Solution
|
- To increase significantly the concentration of free Zn^(2+) ion is a s...
Text Solution
|
- CoS (black) obtained in group IV of salt analysis is dissolved in aqua...
Text Solution
|
- A metal salt solution when treated with dimethyl glyoxime and NH(4)OH ...
Text Solution
|
- An aqueous solution of colourless metal sulphate M, gives a white prec...
Text Solution
|