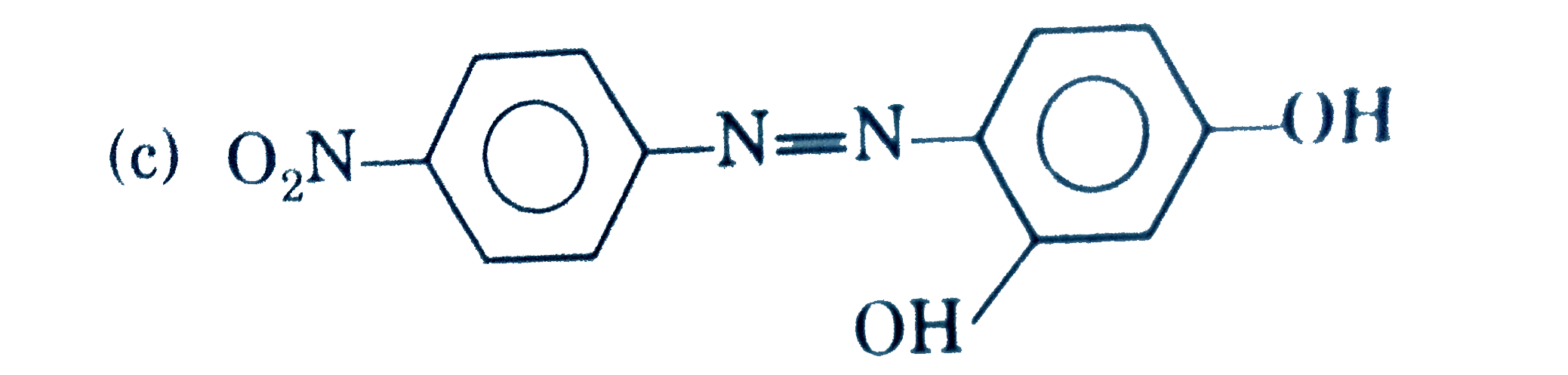
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
SALT ANALYSIS
GRB PUBLICATION|Exercise L: Miscellaneous|40 VideosSALT ANALYSIS
GRB PUBLICATION|Exercise Reasoning Type|49 VideosSALT ANALYSIS
GRB PUBLICATION|Exercise J: IVth Group Cations|24 VideosS BLOCK ELEMENTS
GRB PUBLICATION|Exercise SUBJECTIVE TYPE|9 VideosTHERMODYNAMICS
GRB PUBLICATION|Exercise All Questions|704 Videos
Similar Questions
Explore conceptually related problems
GRB PUBLICATION-SALT ANALYSIS-K: Vth and Vth Group Cations
- A metal salt solution forms a yellow precipitate with potassium chroma...
Text Solution
|
- In fifth group, (NH(4))(2)CO(3) is added to precipitate out the carbon...
Text Solution
|
- Select the correct statement with respect to Ca^(2+) ions.
Text Solution
|
- The solid laboratory reagent A gives the following reactions: I. It ...
Text Solution
|
- Aqueous solution of BaBr(2) gives yellow precipitate with:
Text Solution
|
- The addition of K(2)CO(3)(aq) to the following solution is expected to...
Text Solution
|
- An aqueous solution of salt gives salt precipitate with AgNO(3) soluti...
Text Solution
|
- The presence of magnesium is confirmed in the qualitative analysis by:
Text Solution
|
- Mg is not precipitated in V group because:
Text Solution
|
- MgSO(4) on reaction with NH(4)OH and Na(2)HPO(4) forms a white crystal...
Text Solution
|