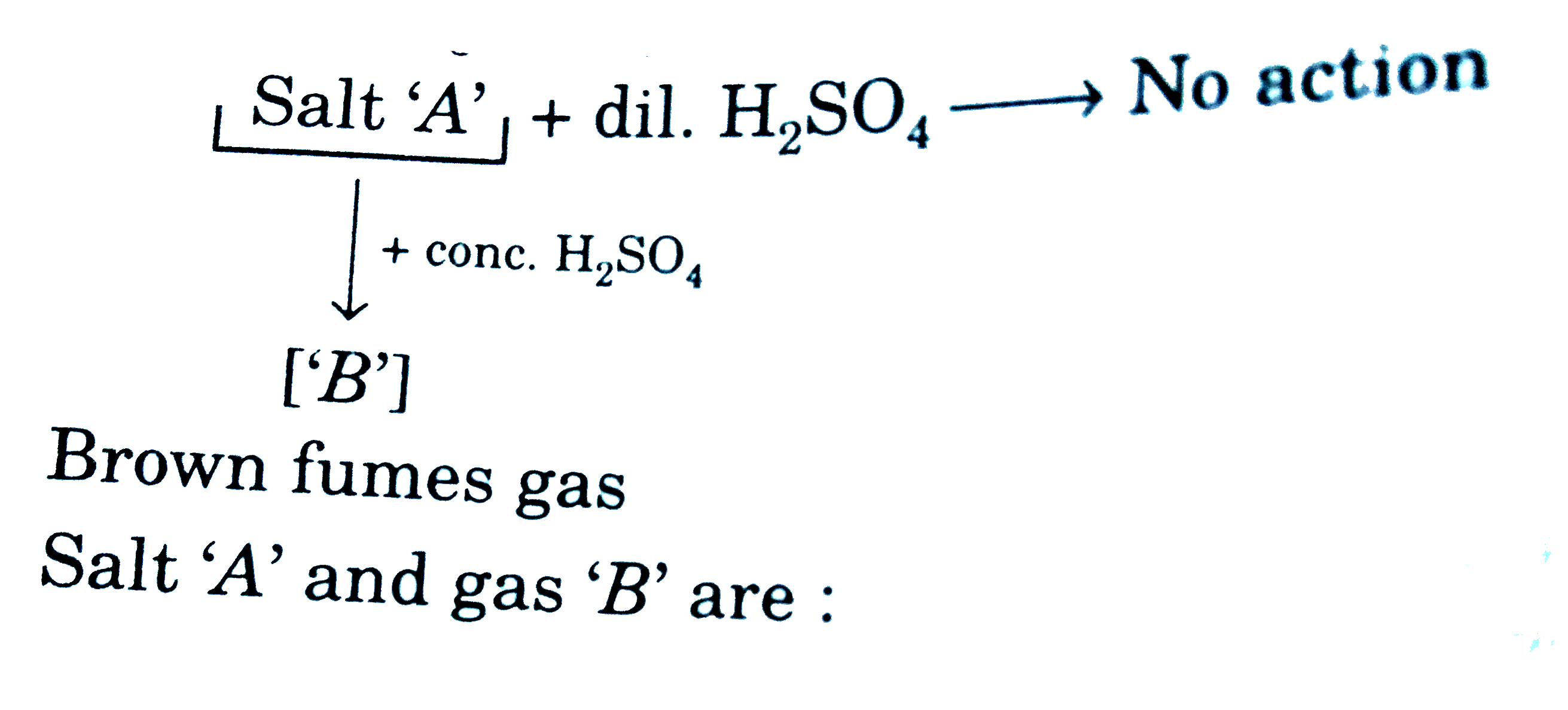A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
SALT ANALYSIS
GRB PUBLICATION|Exercise Reasoning Type|49 VideosSALT ANALYSIS
GRB PUBLICATION|Exercise Multiple Objective Type|129 VideosSALT ANALYSIS
GRB PUBLICATION|Exercise K: Vth and Vth Group Cations|10 VideosS BLOCK ELEMENTS
GRB PUBLICATION|Exercise SUBJECTIVE TYPE|9 VideosTHERMODYNAMICS
GRB PUBLICATION|Exercise All Questions|704 Videos
Similar Questions
Explore conceptually related problems
GRB PUBLICATION-SALT ANALYSIS-L: Miscellaneous
- When 'A' salt solution is added carefully to a saturated solution of F...
Text Solution
|
- An inorganic salt solution 'M' gives white precipitate with Pb(OAc)(2)...
Text Solution
|
- The mixture of salts NaCI,NaBr, NaI on adding conc. H(2)SO(4) and foll...
Text Solution
|
- Compound 'A' and 'C' may be:
Text Solution
|
- Aq. Solution of 'A' overset(Na(2)S(2)O(3))underset("solution")rarr vio...
Text Solution
|
- Brown fumes gas Salt 'A' and gas 'B' are:
Text Solution
|
- Brown fumes of 'X' + Blue coloured solution of 'Y' Here 'Y' is:
Text Solution
|
- Concentrated solution of which salt gives white precipitate with ferri...
Text Solution
|
- Choose the correct statements from the following:
Text Solution
|
- Which of the following compound is not soluble in conc. (50%) HNO(3)?
Text Solution
|
- Silver is not produced when:
Text Solution
|
- Which of the following compound gives a black precipitate with ammonia...
Text Solution
|
- Which of the following cations give black precipitate with H(2)S gas a...
Text Solution
|
- Here, white ppt. of ['Y'] is:
Text Solution
|
- Addition of SnCI(2) to HgCI(2) gives a ppt. which is:
Text Solution
|
- A metal ion 'X' reacts with NaOH to give a white ppt. precipitate can ...
Text Solution
|
- Which of the following metal sulphide is soluble in hot and conc. HNO(...
Text Solution
|
- Which of the following metal cation gives yellow ppt. with pyrogallol ...
Text Solution
|
- With 'X' cation [Fe(CN)(6)]^(4-) gives prussian blue colouration due t...
Text Solution
|
- Turnbull's blue is formed when Fe^(+2) ions are added to K(3)[Fe(CN)(6...
Text Solution
|