Text Solution
Verified by Experts
Topper's Solved these Questions
CHEMICAL BONDING AND MOLECULAR STRUCTURE
DINESH PUBLICATION|Exercise BORD EXAMINATIONS|84 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
DINESH PUBLICATION|Exercise ADDITIONAL IMPORTANT QUASTIONS|41 VideosCARBOXYLIC ACIDS AND THEIR DERIVATIVES
DINESH PUBLICATION|Exercise Unit test|20 VideosCLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIES
DINESH PUBLICATION|Exercise All Questions|310 Videos
Similar Questions
Explore conceptually related problems
DINESH PUBLICATION-CHEMICAL BONDING AND MOLECULAR STRUCTURE -COMPREHENSION
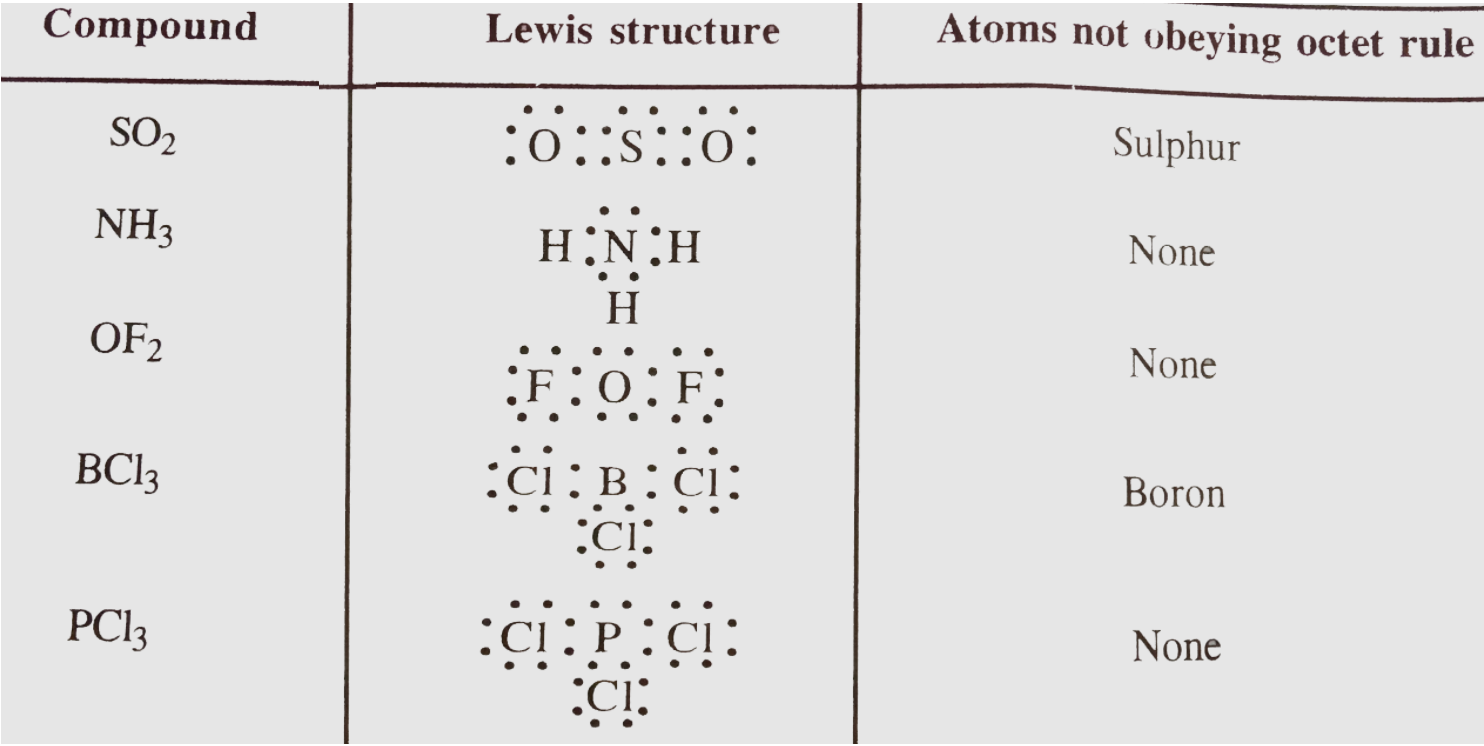
- Identify the atoms in each of the following compounds which do not obe...
Text Solution
|
- Molecular orbitals are formed by the overlap of atomic orbitals. Two a...
Text Solution
|
- Molecular orbitals are formed by the overlap of atomic orbitals. Two a...
Text Solution
|
- Molecular orbitals are formed by the overlap of atomic orbitals. Two a...
Text Solution
|
- Molecular orbitals are formed by the overlap of atomic orbitals. Two a...
Text Solution
|