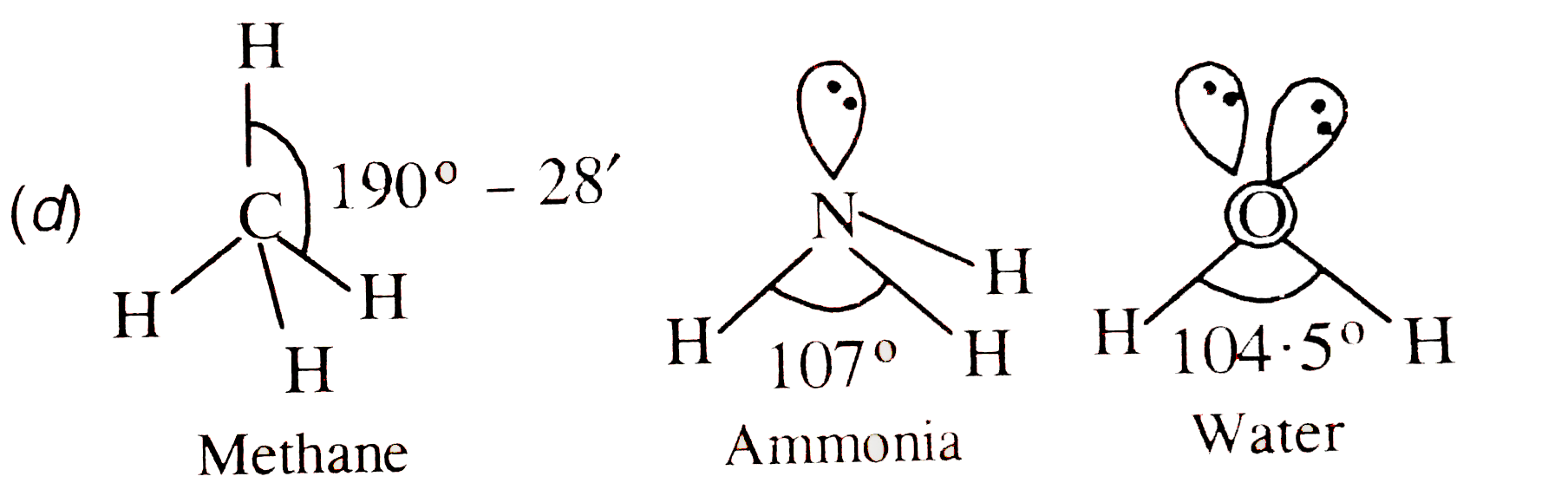A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL BONDING AND MOLECULAR STRUCTURE
DINESH PUBLICATION|Exercise JEE (MAIN) & OTHER ENGINEERING ENTRANCE EXAMINATIONS|46 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
DINESH PUBLICATION|Exercise JEE (JOINT ENTRANGE EXAMINATION) ADVANCED COMPREHENSION LINKED MCQs|34 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
DINESH PUBLICATION|Exercise HYDROGEN BONDING|10 VideosCARBOXYLIC ACIDS AND THEIR DERIVATIVES
DINESH PUBLICATION|Exercise Unit test|20 VideosCLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIES
DINESH PUBLICATION|Exercise All Questions|310 Videos
Similar Questions
Explore conceptually related problems
DINESH PUBLICATION-CHEMICAL BONDING AND MOLECULAR STRUCTURE -MULTIPLE CHOICE QUESTIONS BANK (MCQB)
- During change of O(2) to O(2)^(2-) ion, the electrons add on which of ...
Text Solution
|
- Which one of the following molecules contains no pi - bond ?
Text Solution
|
- XeF(2) is iso-structural with :
Text Solution
|
- Dipole-induced dipole interaction are present in which of the followin...
Text Solution
|
- Which of the following is paramagnetic?
Text Solution
|
- Which of the following species has plane tringular shape ?
Text Solution
|
- Which one of the following is paramagnetic in nature ?
Text Solution
|
- Which of the following pairs of ions are isoelectronic and also isostr...
Text Solution
|
- The correct bond order in the following species is:
Text Solution
|
- The total number of pi-bond electrons in the following structure is
Text Solution
|
- Which of the following species contains equal number of pi and pi bond...
Text Solution
|
- Decreasing order of stability of O(2), O(2)^(-), O(2)^(+) and O(2)^(2-...
Text Solution
|
- Which of the following sets of molecules contains the same number of l...
Text Solution
|
- Which one of the following does not match with respect to the shape o...
Text Solution
|
- Find the pair that has the same bond order with diamagnetic and parama...
Text Solution
|
- In which one of the following compounds does the central atom obey the...
Text Solution
|
- Consider the molecules CH(4),NH(3) and H(2)O which of the given statem...
Text Solution
|
- Predict the correct order omong the following:
Text Solution
|
- The hybridisatipon of atomic orbitals of nitrogen in NO(2)^(+),NO(3)^(...
Text Solution
|
- The species with fractional bond order is :
Text Solution
|