A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL BONDING AND MOLECULAR STRUCTURE
DINESH PUBLICATION|Exercise STRAIGHT OBJECTIVE TYPE MCQs (SINGLE CORRECT OPTION)|1 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
DINESH PUBLICATION|Exercise MULTIPLE CHOICE ANSWER TYPE MCQs|5 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
DINESH PUBLICATION|Exercise JEE (MAIN) & OTHER ENGINEERING ENTRANCE EXAMINATIONS|46 VideosCARBOXYLIC ACIDS AND THEIR DERIVATIVES
DINESH PUBLICATION|Exercise Unit test|20 VideosCLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIES
DINESH PUBLICATION|Exercise All Questions|310 Videos
Similar Questions
Explore conceptually related problems
DINESH PUBLICATION-CHEMICAL BONDING AND MOLECULAR STRUCTURE -JEE (JOINT ENTRANGE EXAMINATION) ADVANCED COMPREHENSION LINKED MCQs
- Which one among the following does not have the hydrogen bond?
Text Solution
|
- The bond between two identical non-metal atoms has a pair of electrons...
Text Solution
|
- Hybridisationof sulphur in So(2) is :
Text Solution
|
- The melecule that has linear structure is:
Text Solution
|
- The type of hybrid orbitals used by the chlorine atom in CIO(2^(-)) is
Text Solution
|
- The maximum possible number of hydrogen bonds a water molecule can for...
Text Solution
|
- The number and type of bonds between two carbon atoms in CaC(2) are:
Text Solution
|
- The cyanide ion CN and N(2) are isoelectronic, but in contrast to CN^(...
Text Solution
|
- The geometry of H2 S and its dipole moment are :
Text Solution
|
- The hybridization of atomic orbitals of nitrogen is NO(2)^(+), NO(3)^(...
Text Solution
|
- The common features among the species CN^- , CO and NO^+ are :
Text Solution
|
- Identify the least stable among the following
Text Solution
|
- Which of the following are iso-electronic as well as is structural ? ...
Text Solution
|
- According to MO theory,
Text Solution
|
- The bond angle in H(2)S "is" 92^(@). It suggest that :
Text Solution
|
- The species having bond order different from that in CO is
Text Solution
|
- The bond energy (in kcal mol^(-1)) of a C -c single bond is approximat...
Text Solution
|
- In allene (C(3)H(4)), the type (s) of the carbon atom (s) is (are) :
Text Solution
|
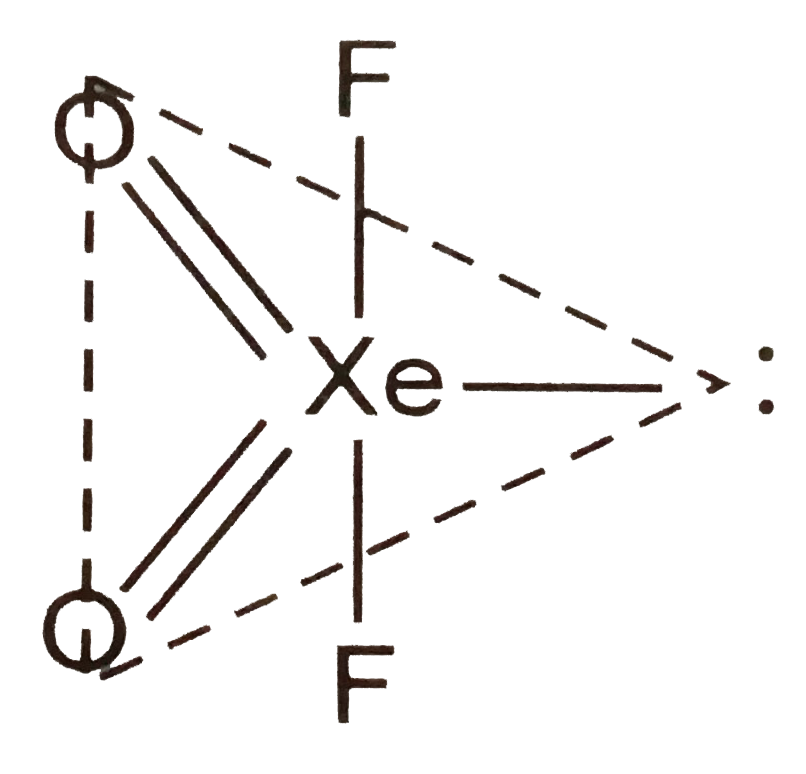
- The shapes of XeO(2)F(2) molecule is
Text Solution
|
- Assuming 2s-2p mixing is NOT operative, the paramagnetic species among...
Text Solution
|